Search for drugs:
Typing the drug name to query
TRABECTEDIN
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of trabectedin on the QT/QTc interval was evaluated in 75 patients who received placebo on day 1 and trabectedin (1.3 mg/m2) as a 3-hour intravenous infusion on day 2. No patients in the study showed a QTc interval exceeding 500 msec or more than 60 msec increase from baseline, and no large changes in the mean QTc interval (i.e., >20 msec) were observed.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
39
42873
Other ADRs
613
14116666
Odds Ratio = 20.949
Drug Property Information
ATC Code(s):
- L01CX01 - trabectedin
- L01CX - Other plant alkaloids and natural products
- L01C - PLANT ALKALOIDS AND OTHER NATURAL PRODUCTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:trabectedin
Active Ingredient UNII:ID0YZQ2TCP
Drugbank ID:DB05109
PubChem Compound:108150
CAS Number:114899-77-3
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 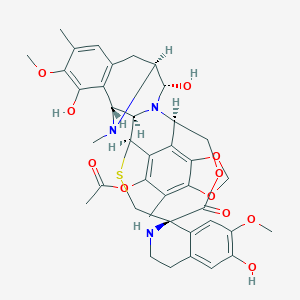

SMILE Code:
CC1=C(C(=C2[C@@H]3[C@@H]4[C@H]5C6=C([C@@H](N4[C@H]([C@@H](N3C)CC2=C1)O)COC(=O)[C@@]7(CS5)C8=CC(=C(C=C8CCN7)O)OC)C9=C(C(=C6OC(=O)C)C)OCO9)O)OC
CC1=C(C(=C2[C@@H]3[C@@H]4[C@H]5C6=C([C@@H](N4[C@H]([C@@H](N3C)CC2=C1)O)COC(=O)[C@@]7(CS5)C8=CC(=C(C=C8CCN7)O)OC)C9=C(C(=C6OC(=O)C)C)OCO9)O)OC
Reference
COHORT STUDY:
1: Trabectedin for inoperable or recurrent soft tissue sarcoma in adult patients: a retrospective cohort study.
[Angarita FA, Cannell AJ, Abdul Razak AR, Dickson BC, Blackstein ME, BMC Cancer. 2016 Jan 19;16:30.]ABSTRACT
BACKGROUND: Trabectedin is an antineoplastic agent used for patients with soft tissue sarcoma (STS) who fail standard-of-care treatment. Real-world data of its performance is scarce. This study evaluates the safety and effectiveness of trabectedin for patients with advanced STS who were treated at a high-volume sarcoma center.
METHODS: A retrospective chart review was performed on 77 patients treated with trabectedin (24 h infusion q3w) between 01/2005 and 05/2014. Data regarding safety, objective radiological response, progression-free and overall survival were analyzed.
RESULTS: Median age at treatment onset was 52y [interquartile range (IQR): 45-61y]. Tumors included leiomyosarcoma (41.6%), liposarcoma (18.2%), and synovial sarcoma (13%). Trabectedin was provided as ≥ third-line chemotherapy in 71.4%. Median number of cycles was 2 (range: 1-17). Dose reduction and treatment delays occurred in 19.5 and 40.3%, respectively. Toxicities occurred in 78%, primarily for neutropenia or elevated liver enzymes. Two patients died secondary to trabectedin-induced rhabdomyolysis. Treatment was discontinued because of disease progression (84.7%), toxicity (10%), and patient preference (5%). Partial response or stable disease occurred in 14.1 and 33.8%, respectively, while 52.1% developed progressive disease. Median progression-free survival was 1.3 m (IQR: 0.7-3.5 m) and was significantly higher in patients lacking severe toxicities or progressive disease. Median overall survival was 6.7 m (IQR: 2.3-12.7 m) and was significantly higher in patients with leiomyosarcoma or liposarcoma relative to other histologies.
CONCLUSIONS: Trabectedin has an acceptable safety profile as an anti-tumor agent. Our data further suggest there may be some benefit in using trabectedin particularly in patients with leiomyo- or liposarcoma who failed standard-of-care agents.
PMID: 26786213
METHODS: A retrospective chart review was performed on 77 patients treated with trabectedin (24 h infusion q3w) between 01/2005 and 05/2014. Data regarding safety, objective radiological response, progression-free and overall survival were analyzed.
RESULTS: Median age at treatment onset was 52y [interquartile range (IQR): 45-61y]. Tumors included leiomyosarcoma (41.6%), liposarcoma (18.2%), and synovial sarcoma (13%). Trabectedin was provided as ≥ third-line chemotherapy in 71.4%. Median number of cycles was 2 (range: 1-17). Dose reduction and treatment delays occurred in 19.5 and 40.3%, respectively. Toxicities occurred in 78%, primarily for neutropenia or elevated liver enzymes. Two patients died secondary to trabectedin-induced rhabdomyolysis. Treatment was discontinued because of disease progression (84.7%), toxicity (10%), and patient preference (5%). Partial response or stable disease occurred in 14.1 and 33.8%, respectively, while 52.1% developed progressive disease. Median progression-free survival was 1.3 m (IQR: 0.7-3.5 m) and was significantly higher in patients lacking severe toxicities or progressive disease. Median overall survival was 6.7 m (IQR: 2.3-12.7 m) and was significantly higher in patients with leiomyosarcoma or liposarcoma relative to other histologies.
CONCLUSIONS: Trabectedin has an acceptable safety profile as an anti-tumor agent. Our data further suggest there may be some benefit in using trabectedin particularly in patients with leiomyo- or liposarcoma who failed standard-of-care agents.
OTHER REFERENCE(S):
1: FDA Approval Summary: Trabectedin for Unresectable or Metastatic Liposarcoma or Leiomyosarcoma Following an Anthracycline-Containing Regimen.
[Barone Amy,Chi Dow-Chung,Theoret Marc R,Chen Huanyu,He Kun,Kufrin Dubravka,Helms Whitney S,Subramaniam Sriram,Zhao Hong,Patel Anuja,Goldberg Kirsten B,Keegan Patricia,Pazdur Richard]Clin Cancer Res.2017 Dec 15;23(24):7448-7453. doi: 10.1158/1078-0432.CCR-17-0898. Epub 2017 Aug 3. PMID: 28774898
2: Severe Rhabdomyolysis during Treatment with Trabectedin in Combination with a Herbal Drug in a Patient with Metastatic Synovial Sarcoma: A Case Report.
[Damato Angela,Larocca Mario,Rondini Ermanno,Menga Massimo,Pinto Carmine,Versari Annibale]Case Rep Oncol.2017 Mar 28;10(1):258-264. doi: 10.1159/000464440. eCollection 2017 Jan-Apr. PMID: 28512407
3: Trabectedin for Soft Tissue Sarcoma: Current Status and Future Perspectives.
[Gordon Erlinda M,Sankhala K Kumar,Chawla Neal,Chawla Sant P]Adv Ther.2016 Jul;33(7):1055-71. doi: 10.1007/s12325-016-0344-3. Epub 2016 May 27. PMID: 27234989
4: Trabectedin for inoperable or recurrent soft tissue sarcoma in adult patients: a retrospective cohort study.
[Angarita Fernando A,Cannell Amanda J,Abdul Razak Albiruni R,Dickson Brendan C,Blackstein Martin E]BMC Cancer.2016 Jan 19;16:30. doi: 10.1186/s12885-016-2054-2. PMID: 26786213
5: Safety evaluation of trabectedin in treatment of soft-tissue sarcomas.
[Martin-Liberal Juan,Judson Ian]Expert Opin Drug Saf.2013 Nov;12(6):905-11. doi: 10.1517/14740338.2013.829037. Epub 2013 Aug 12. PMID: 23937190
6: Herbal-drug interaction induced rhabdomyolysis in a liposarcoma patient receiving trabectedin.
[Strippoli Sabino,Lorusso Vito,Albano Anna,Guida Michele]BMC Complement Altern Med.2013 Jul 30;13:199. doi: 10.1186/1472-6882-13-199. PMID: 23899130
7: A comprehensive safety analysis confirms rhabdomyolysis as an uncommon adverse reaction in patients treated with trabectedin.
[Grosso Federica,D'Incalci Maurizio,Cartoafa Mirela,Nieto Antonio,Fernández-Teruel Carlos,Alfaro Vicente,Lardelli Pilar,Roy Elena,Gómez Javier,Kahatt Carmen,Soto-Matos Arturo,Judson Ian]Cancer Chemother Pharmacol.2012 Jun;69(6):1557-65. doi: 10.1007/s00280-012-1864-4. Epub 2012 Apr 7. PMID: 22484722
8: Problems in dealing with very rare adverse effects of new anticancer drugs: the example of trabectedin.
[Grosso Federica,D'Incalci Maurizio]Tumori.2011 Mar-Apr;97(2):256. doi: 10.1700/667.7796. PMID: 21617728
9: Trabectedin-related rhabdomyolysis: an uncommon but fatal toxicity.
[Stoyianni Aikaterini,Kapodistrias Nikolaos,Kampletsas Eleutherios,Pentheroudakis George,Pavlidis Nicholas]Tumori.2011 Mar-Apr;97(2):252-5. doi: 10.1700/667.7795. PMID: 21617727
10: Case Report of Suspected Rhabdomyolysis during Treatment with Trabectedin in a Patient with Metastatic Leiomyosarcoma.
[Lamm W,Amann G,Brodowicz T]Case Rep Oncol.2010 Sep;3(3):477-9. doi: 10.1159/000323261. Epub 2010 Dec 15. PMID: 21611101
11: A retrospective pooled analysis of trabectedin safety in 1,132 patients with solid tumors treated in phase II clinical trials.
[Le Cesne Axel,Yovine Alejandro,Blay Jean-Yves,Delaloge Suzette,Maki Robert G,Misset Jean-Louis,Frontelo Pilar,Nieto Antonio,Jiao Juhui James,Demetri George D]Invest New Drugs.2012 Jun;30(3):1193-202. doi: 10.1007/s10637-011-9662-0. Epub 2011 Apr 12. PMID: 21484250
12: Fatal rhabdomyolysis as a complication of ET-743 (Yondelis) chemotherapy for sarcoma.
[Skorupa Amy,Beldner Matthew,Kraft Andrew,Montero Alberto J]Cancer Biol Ther.2007 Jul;6(7):1015-7. PMID: 17611408
13: Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients.
[Yovine A,Riofrio M,Blay J Y,Brain E,Alexandre J,Kahatt C,Taamma A,Jimeno J,Martin C,Salhi Y,Cvitkovic E,Misset J L]J Clin Oncol.2004 Mar 1;22(5):890-9. PMID: 14990645
14: Safety and efficacy of ET-743: the French experience.
[Brain Etienne G C]Anticancer Drugs.2002 May;13 Suppl 1:S11-4. PMID: 12173489
15: Phase I and pharmacokinetic study of ecteinascidin 743 administered as a 72-hour continuous intravenous infusion in patients with solid malignancies.
[Ryan D P,Supko J G,Eder J P,Seiden M V,Demetri G,Lynch T J,Fischman A J,Davis J,Jimeno J,Clark J W]Clin Cancer Res.2001 Feb;7(2):231-42. PMID: 11234874
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.