Search for drugs:
Typing the drug name to query
PITAVASTATIN CALCIUM
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, double-blind, placebo-controlled, 4-way parallel, active-comparator study with moxifloxacin in 174 healthy participants, LIVALO was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to 16 mg (4 times the recommended maximum dose of 4 mg daily).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
104
42808
Other ADRs
902
14116377
Odds Ratio = 38.022
Drug Property Information
ATC Code(s):
- C10AA08 - pitavastatin calcium
- C10AA - HMG CoA reductase inhibitors
- C10A - "LIPID MODIFYING AGENTS, PLAIN"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:pitavastatin calcium
Active Ingredient UNII:IYD54XEG3W
Drugbank ID:DB08860
PubChem Compound:5282452
CAS Number:147511-69-1
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 2.0 mg/day C10AA08
Chemical Structure: 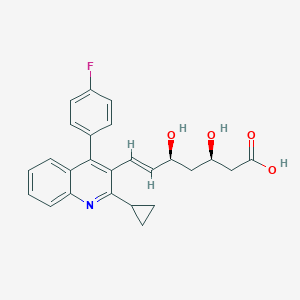

SMILE Code:
C1CC1C2=NC3=CC=CC=C3C(=C2/C=C/[C@H](C[C@H](CC(=O)O)O)O)C4=CC=C(C=C4)F
C1CC1C2=NC3=CC=CC=C3C(=C2/C=C/[C@H](C[C@H](CC(=O)O)O)O)C4=CC=C(C=C4)F
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.