Search for drugs:
Typing the drug name to query
SUNITINIB MALATE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation and Torsade de Pointes
- SUTENT can cause QT interval prolongation in a dose-dependent manner, which may lead to an increased risk for ventricular arrhythmias including Torsade de Pointes. Torsade de Pointes was observed in <0.1% of patients.
- Monitor patients who are at higher risk of developing QT interval prolongation, including patients with a history of QT interval prolongation, patients who are taking antiarrhythmics, or patients with relevant pre-existing cardiac disease, bradycardia, or electrolyte disturbances. Consider periodic monitoring of electrocardiograms and electrolytes (i.e., magnesium, potassium) during treatment with SUTENT.
- Monitor QT interval more frequently when SUTENT is concomitantly administered with strong CYP3A4 inhibitors or drugs known to prolong QT interval. Consider dose reducing SUTENT [see DOSAGE AND ADMINISTRATION (2.5), DRUG INTERACTIONS (7.2)].
- DRUG INTERACTIONS
- Drugs that Prolong QT Interval
- SUTENT is associated with QTc interval prolongation [see WARNINGS AND PRECAUTIONS (5.3), CLINICAL PHARMACOLOGY (12.2)]. Monitor the QT interval with ECGs more frequently in patients who require treatment with concomitant medications known to prolong the QT interval.
- ADVERSE REACTIONS
- The following clinically significant adverse reactions are described elsewhere in the labeling.
- Hepatotoxicity [see WARNINGS AND PRECAUTIONS (5.1)]
- Cardiovascular Events [see WARNINGS AND PRECAUTIONS (5.2)]
- QT Interval Prolongation and Torsade de Pointes [see WARNINGS AND PRECAUTIONS (5.3)]
- Hypertension [see WARNINGS AND PRECAUTIONS (5.4)]
- Hemorrhagic Events [see WARNINGS AND PRECAUTIONS (5.5)]
- Tumor Lysis Syndrome [see WARNINGS AND PRECAUTIONS (5.6)]
- Thrombotic Microangiopathy [see WARNINGS AND PRECAUTIONS (5.7)]
- Proteinuria [see WARNINGS AND PRECAUTIONS (5.8)]
- Dermatologic Toxicities [see WARNINGS AND PRECAUTIONS (5.9)]
- Reversible Posterior Leukoencephalopathy Syndrome [see WARNINGS AND PRECAUTIONS (5.10)]
- Thyroid Dysfunction [see WARNINGS AND PRECAUTIONS (5.11)]
- Hypoglycemia [see WARNINGS AND PRECAUTIONS (5.12)]
- Osteonecrosis of the Jaw [see WARNINGS AND PRECAUTIONS (5.13)]
- Impaired Wound Healing [see WARNINGS AND PRECAUTIONS (5.14)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- SUTENT can cause QT interval prolongation in a dose-dependent manner, which may lead to an increased risk for ventricular arrhythmias including Torsade de Pointes [see WARNINGS AND PRECAUTIONS (5.3)].
- PATIENT COUNSELING INFORMATION
- QT Prolongation and Torsade de Pointes
- Inform patients of the signs and symptoms of QT prolongation. Advise patients to contact their healthcare provider immediately in the event of syncope, pre-syncopal symptoms, and cardiac palpitations [see WARNINGS AND PRECAUTIONS (5.3)].
- MEDICATION GUIDE
- SUTENT may cause serious side effects, including:
- Abnormal heart rhythm changes. Changes in the electrical activity of your heart called QT prolongation can cause irregular heart beats that can be life threatening. Your healthcare provider may do electrocardiograms and blood tests (electrolytes) to watch for these problems during your treatment with SUTENT. Tell your healthcare provider immediately if you feel dizzy, faint, or have abnormal heartbeats during your treatment with SUTENT
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
68
42844
Other ADRs
36223
14081056
Odds Ratio = 0.617
Drug Property Information
ATC Code(s):
- L01XE04 - sunitinib malate
- L01XE - Protein kinase inhibitors
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:sunitinib malate
Active Ingredient UNII:LVX8N1UT73
Drugbank ID:DB01268
PubChem Compound:5329102
CAS Number:557795-19-4
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 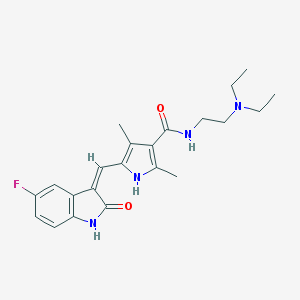

SMILE Code:
CCN(CC)CCNC(=O)C1=C(NC(=C1C)/C=C\2/C3=C(C=CC(=C3)F)NC2=O)C
CCN(CC)CCNC(=O)C1=C(NC(=C1C)/C=C\2/C3=C(C=CC(=C3)F)NC2=O)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.