Search for drugs:
Typing the drug name to query
OXALIPLATIN
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation and Ventricular Arrhythmias
- QT prolongation and ventricular arrhythmias, including fatal torsade de pointes, have been reported with oxaliplatin injection [see Adverse Reactions (6.2)].
- Avoid oxaliplatin injection in patients with congenital long QT syndrome. Monitor electrocardiograms (ECG) in patients with congestive heart failure, bradyarrhythmias, and electrolyte abnormalities and in patients taking drugs known to prolong the QT interval, including Class Ia and III antiarrhythmics [see Drug Interactions (7.1)]. Monitor and correct electrolyte abnormalities prior to initiating oxaliplatin injection and periodically during treatment.
- DRUG INTERACTIONS
- Drugs that Prolong the QT Interval
- QT interval prolongation and ventricular arrhythmias can occur with oxaliplatin injection [see Warnings and Precautions (5.7)]. Avoid coadministration of oxaliplatin injection with medicinal products with a known potential to prolong the QT interval.
- ADVERSE REACTIONS
- QT Interval Prolongation and Ventricular Arrhythmias [see Warnings and Precautions (5.7)]
- The following adverse reactions have been identified during postapproval use of oxaliplatin injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular: QT prolongation leading to ventricular arrhythmias, including fatal torsade de pointes; bradyarrhythmia
- PATIENT COUNSELING INFORMATION
- QT Interval Prolongation
- Advise patients that oxaliplatin injection can cause QTc interval prolongation and to inform their physician if they have any symptoms, such as syncope [see Warnings and Precautions (5.7)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
29
42883
Other ADRs
14801
14102478
Odds Ratio = 0.645
Drug Property Information
ATC Code(s):
- L01XA03 - oxaliplatin
- L01XA - Platinum compounds
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:oxaliplatin
Active Ingredient UNII:04ZR38536J
Drugbank ID:DB00526
PubChem Compound:6857599
CAS Number:61825-94-3
Dosage Form(s):injection, solution, concentrate
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 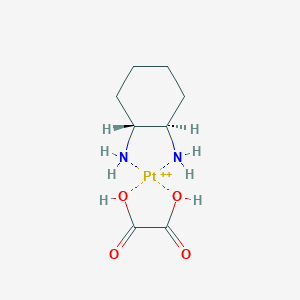

SMILE Code:
C1CC[C@H]([C@@H](C1)N)N.C(=O)(C(=O)O)O.[Pt+2]
C1CC[C@H]([C@@H](C1)N)N.C(=O)(C(=O)O)O.[Pt+2]
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [A Case of Rhabdomyolysis Related to SOX Therapy for Liver Metastasis of Gastric Cancer].
[Sato Kei,Akiyama Hirotoshi,Kogure Yuu,Suwa Yusuke,Momiyama Masashi,Ishibe Atsushi,Endo Itaru]Gan To Kagaku Ryoho.2017 Apr;44(4):329-331. PMID: 28428515
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.