Search for drugs:
Typing the drug name to query
CYCLOPHOSPHAMIDE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified from clinical trials or post-marketing surveillance. Because they are reported from a population from unknown size, precise estimates of frequency cannot be made.
- Cardiac: cardiac arrest, ventricular fibrillation, ventricular tachycardia, cardiogenic shock, pericardial effusion (progressing to cardiac tamponade), myocardial hemorrhage, myocardial infarction, cardiac failure (including fatal outcomes), cardiomyopathy, myocarditis, pericarditis, carditis, atrial fibrillation, supraventricular arrhythmia, ventricular arrhythmia, bradycardia, tachycardia, palpitations, QT prolongation.
- Congenital, Familial and Genetic: intra-uterine death, fetal malformation, fetal growth retardation, fetal toxicity (including myelosuppression, gastroenteritis).
- Ear and Labyrinth: deafness, hearing impaired, tinnitus.
- Endocrine: water intoxication.
- Eye: visual impairment, conjunctivitis, lacrimation.
- Gastrointestinal: gastrointestinal hemorrhage, acute pancreatitis, colitis, enteritis, cecitis, stomatitis, constipation, parotid gland inflammation.
- General Disorders and Administrative Site Conditions: multiorgan failure, general physical deterioration, influenza-like illness, injection/infusion site reactions (thrombosis, necrosis, phlebitis, inflammation, pain, swelling, erythema), pyrexia, edema, chest pain, mucosal inflammation, asthenia, pain, chills, fatigue, malaise, headache.
- Hematologic: myelosuppression, bone marrow failure, disseminated intravascular coagulation and hemolytic uremic syndrome (with thrombotic microangiopathy).
- Hepatic: veno-occlusive liver disease, cholestatic hepatitis, cytolytic hepatitis, hepatitis, cholestasis; hepatotoxicity with hepatic failure, hepatic encephalopathy, ascites, hepatomegaly, blood bilirubin increased, hepatic function abnormal, hepatic enzymes increased.
- Immune: immunosuppression, anaphylactic shock and hypersensitivity reaction.
- Infections: The following manifestations have been associated with myelosuppression and immunosuppression caused by cyclophosphamide: increased risk for and severity of pneumonias (including fatal outcomes), other bacterial, fungal, viral, protozoal and, parasitic infections; reactivation of latent infections, (including viral hepatitis, tuberculosis), Pneumocystis jiroveci, herpes zoster, Strongyloides, sepsis and septic shock.
- Investigations: blood lactate dehydrogenase increased, C-reactive protein increased.
- Metabolism and Nutrition: hyponatremia, fluid retention, blood glucose increased, blood glucose decreased.
- Musculoskeletal and Connective Tissue: rhabdomyolysis, scleroderma, muscle spasms, myalgia, arthralgia.
- Neoplasms: acute leukemia, myelodysplastic syndrome, lymphoma, sarcomas, renal cell carcinoma, renal pelvis cancer, bladder cancer, ureteric cancer, thyroid cancer.
- Nervous System: encephalopathy, convulsion, dizziness, neurotoxicity has been reported and manifested as reversible posterior leukoencephalopathy syndrome, myelopathy, peripheral neuropathy, polyneuropathy, neuralgia, dysesthesia, hypoesthesia, paresthesia, tremor, dysgeusia, hypogeusia, parosmia.
- Pregnancy: premature labor.
- Psychiatric: confusional state.
- Renal and Urinary: renal failure, renal tubular disorder, renal impairment, nephropathy toxic, hemorrhagic cystitis, bladder necrosis, cystitis ulcerative, bladder contracture, hematuria, nephrogenic diabetes insipidus, atypical urinary bladder epithelial cells.
- Reproductive System: infertility, ovarian failure, ovarian disorder, amenorrhea, oligomenorrhea, testicular atrophy, azoospermia, oligospermia.
- Respiratory: pulmonary veno-occlusive disease, acute respiratory distress syndrome, interstitial lung disease as manifested by respiratory failure (including fatal outcomes), obliterative bronchiolitis, organizing pneumonia, alveolitis allergic, pneumonitis, pulmonary hemorrhage; respiratory distress, pulmonary hypertension, pulmonary edema, pleural effusion, bronchospasm, dyspnea, hypoxia, cough, nasal congestion, nasal discomfort, oropharyngeal pain, rhinorrhea.
- Skin and Subcutaneous Tissue: toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme, palmar-plantar erythrodysesthesia syndrome, radiation recall dermatitis, toxic skin eruption, urticaria, dermatitis, blister, pruritus, erythema, nail disorder, facial swelling, hyperhidrosis.
- Tumor lysis syndrome: like other cytotoxic drugs, cyclophosphamide may induce tumor-lysis syndrome and hyperuricemia in patients with rapidly growing tumors.
- Vascular: pulmonary embolism, venous thrombosis, vasculitis, peripheral ischemia, hypertension, hypotension, flushing, hot flush.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
26
42886
Other ADRs
22346
14094933
Odds Ratio = 0.383
Drug Property Information
ATC Code(s):
- L01AA01 - cyclophosphamide
- L01AA - Nitrogen mustard analogues
- L01A - ALKYLATING AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:cyclophosphamide
Active Ingredient UNII:8N3DW7272P
Drugbank ID:DB00531
PubChem Compound:2907
CAS Number:50-18-0
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous; oral
Daily Dose:
Chemical Structure: 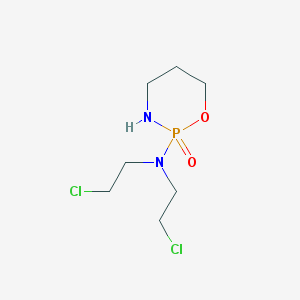

SMILE Code:
C1CNP(=O)(OC1)N(CCCl)CCCl
C1CNP(=O)(OC1)N(CCCl)CCCl
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rhabdomyolysis in a Patient with Polyarteritis Nodosa.
[Iida Harunobu,Hanaoka Hironari,Asari Yusa,Ishimori Kana,Kiyokawa Tomofumi,Takakuwa Yukiko,Yamasaki Yoshioki,Yamada Hidehiro,Okazaki Takahiro,Doi Masatomo,Ozaki Shoichi]Intern Med.2018 Jan 1;57(1):101-106. doi: 10.2169/internalmedicine.8913-17. Epub 2017 Oct 11. PMID: 29021478
2: [Thyroid storm associated with multiorganic dysfunction].
[Baena Juan Camilo,Padilla Jacobo,Guzmán Guillermo]Medicina (B Aires).2017;77(4):337-340. PMID: 28825582
3: Lymphoma in Danon disease with chronic rhabdomyolysis treated with EPOCH-R: A case report.
[Porpaczy Edit,Mayerhoefer Marius,Salzer-Muhar Ulrike,Jaeger Ulrich]Medicine (Baltimore).2016 Jul;95(29):e4237. doi: 10.1097/MD.0000000000004237. PMID: 27442649
4: Association of Progressive Cerebellar Atrophy With Long-term Outcome in Patients With Anti-N-Methyl-d-Aspartate Receptor Encephalitis.
[Iizuka Takahiro,Kaneko Juntaro,Tominaga Naomi,Someko Hidehiro,Nakamura Masaaki,Ishima Daisuke,Kitamura Eiji,Masuda Ray,Oguni Eiichi,Yanagisawa Toshiyuki,Kanazawa Naomi,Dalmau Josep,Nishiyama Kazutoshi]JAMA Neurol.2016 Jun 1;73(6):706-13. doi: 10.1001/jamaneurol.2016.0232. PMID: 27111481
5: Peripheral T-cell lymphoma with unusual clinical presentation of rhabdomyolysis.
[Liu Zhiyu,Medeiros L Jeffrey,Young Ken H]Hematol Oncol.2017 Mar;35(1):125-129. doi: 10.1002/hon.2203. Epub 2015 Apr 28. PMID: 25921311
6: Questionable eponyms: paraparesis and rhabdomyolysis.
[Lowenthal M N,Chorny I,Tishchenko L]Isr Med Assoc J.2013 Sep;15(9):526. PMID: 24340852
7: Movement disorder emergencies in childhood.
[Kirkham F J,Haywood P,Kashyape P,Borbone J,Lording A,Pryde K,Cox M,Keslake J,Smith M,Cuthbertson L,Murugan V,Mackie S,Thomas N H,Whitney A,Forrest K M,Parker A,Forsyth R,Kipps C M]Eur J Paediatr Neurol.2011 Sep;15(5):390-404. doi: 10.1016/j.ejpn.2011.04.005. Epub 2011 Aug 10. PMID: 21835657
8: [Fatal Bacillus cereus sepsis and rhabdomyolysis in a patient with Hodgkin's disease].
[Gómez-Herruz Peña,Gil-Fernández Juan José,Guillen Helga,Arizcorreta Ana]Enferm Infecc Microbiol Clin.2011 Mar;29(3):232-3. doi: 10.1016/j.eimc.2010.06.009. Epub 2011 Feb 15. PMID: 21324561
9: Acute rhabdomyolysis as a complication of cytarabine chemotherapy for acute myeloid leukemia: case report and review of literature.
[Truica Cristina I,Frankel Stanley R]Am J Hematol.2002 Aug;70(4):320-3. PMID: 12210815
10: Acute rhabdomyolysis following administration of high-dose cyclophosphamide: case report.
[Shima E,Hino M,Yamane T,Aoyama Y,Nakamae H,Yamamura R,Makita K,Sugano Y,Yasuda S,Takubo T,Ohta K,Tatsumi N]Ann Hematol.2002 Jan;81(1):55-6. Epub 2001 Dec 11. PMID: 11807638
11: Rhabdomyolysis following administration of cyclophosphamide: a case report in a BMT recipient.
[Tabata N,Tanaka R,Suga S,Mitani Y,Nakano T,Ido M,Azuma E,Ito M,Hamazaki M,Shiraishi T,Sakurai M]Bone Marrow Transplant.1996 Jun;17(6):1167-9. PMID: 8807130
12: Rhabdomyolysis: an unusual complication of cytotoxic chemotherapy.
[Levy R J,Sparano J A,Khan G]Med Oncol.1995 Dec;12(4):219-22. PMID: 8832525
13: Acute rhabdomyolysis complicating viridans streptococcal shock syndrome.
[Martino R,Nomdedéu J,Sureda A,Mateu R,Brunet S,Domingo-Albós A]Acta Haematol.1994;92(3):140-1. PMID: 7871952
14: [Cyclophosphamide and vincristine in therapy of acute kidney failure in idiopathic rhabdomyolysis].
[Bleichner F,Meuret G]Dtsch Med Wochenschr.1980 May 23;105(21):770. PMID: 7379715
15: [Cyclophosphamide and vincristine in therapy of acute renal failure in idiopathic rhabdomyolysis?].
[Schmidt R E]Dtsch Med Wochenschr.1980 May 9;105(19):700-1. PMID: 7371544
16: [Treatment of kidney failure in idiopathic rhabdomyolysis with cortisone, cyclophosphamide and vincristine].
[Bleichner F,Meuret G]Dtsch Med Wochenschr.1980 Mar 28;105(13):451-2. PMID: 7363798
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.