Search for drugs:
Typing the drug name to query
BACLOFEN
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- BOXED WARNING
- Abrupt discontinuation of intrathecal baclofen, regardless of the cause, has resulted in sequelae that include high fever, altered mental status, exaggerated rebound spasticity, and muscle rigidity, that in rare cases has advanced to rhabdomyolysis, multiple organ-system failure and death.
- Prevention of abrupt discontinuation of intrathecal baclofen requires careful attention to programming and monitoring of the infusion system, refill scheduling and procedures, and pump alarms. Patients and caregivers should be advised of the importance of keeping scheduled refill visits and should be educated on the early symptoms of baclofen withdrawal. Special attention should be given to patients at apparent risk (e.g. spinal cord injuries at T-6 or above, communication difficulties, history of withdrawal symptoms from oral or intrathecal baclofen). Consult the technical manual of the implantable infusion system for additional postimplant clinician and patient information (see WARNINGS).
- WARNINGS
- Withdrawal: Abrupt withdrawal of intrathecal baclofen, regardless of the cause, has resulted in sequelae that included high fever, altered mental status, exaggerated rebound spasticity and muscle rigidity that in rare cases progressed to rhabdomyolysis, multiple organ-system failure, and death. In the first 9 years of post-marketing experience, 27 cases of withdrawal temporally related to the cessation of baclofen therapy were reported; six patients died. In most cases, symptoms of withdrawal appeared within hours to a few days following interruption of baclofen therapy. Common reasons for abrupt interruption of intrathecal baclofen therapy included malfunction of the catheter (especially disconnection), low volume in the pump reservoir, and end of pump battery life; human error may have played a causal or contributing role in some cases. Cases of intrathecal mass at the tip of the implanted catheter leading to withdrawal symptoms have also been reported, most of them involving pharmacy compounded analgesic admixtures (see PRECAUTIONS).
- Prevention of abrupt discontinuation of intrathecal baclofen requires careful attention to programming and monitoring of the infusion system, refill scheduling and procedures, and pump alarms. Patients and caregivers should be advised of the importance of keeping scheduled refill visits and should be educated on the early symptoms of baclofen withdrawal.
- All patients receiving intrathecal baclofen therapy are potentially at risk for withdrawal. Early symptoms of baclofen withdrawal may include return of baseline spasticity, pruritus, hypotension, and paresthesias. Priapism may develop or recur if treatment with intrathecal baclofen is interrupted. Some clinical characteristics of the advanced intrathecal baclofen withdrawal syndrome may resemble autonomic dysreflexia, infection (sepsis), malignant hyperthermia, neuroleptic-malignant syndrome, or other conditions associated with a hypermetabolic state or widespread rhabdomyolysis.
- Rapid, accurate diagnosis and treatment in an emergency-room or intensive-care setting are important in order to prevent the potentially life-threatening central nervous system and systemic effects of intrathecal baclofen withdrawal. The suggested treatment for intrathecal baclofen withdrawal is the restoration of intrathecal baclofen at or near the same dosage as before therapy was interrupted. However, if restoration of intrathecal delivery is delayed, treatment with GABA-ergic agonist drugs such as oral or enteral baclofen, or oral, enteral, or intravenous benzodiazepines may prevent potentially fatal sequelae. Oral or enteral baclofen alone should not be relied upon to halt the progression of intrathecal baclofen withdrawal.
- Seizures have been reported during overdose and with withdrawal from LIORESAL INTRATHECAL as well as in patients maintained on therapeutic doses of LIORESAL INTRATHECAL.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
103
42809
Other ADRs
23421
14093858
Odds Ratio = 1.448
Drug Property Information
ATC Code(s):
- M03BX01 - baclofen
- M03BX - Other centrally acting agents
- M03B - "MUSCLE RELAXANTS, CENTRALLY ACTING AGENTS"
- M03 - MUSCLE RELAXANTS
- M - MUSCULO-SKELETAL SYSTEM
Active Ingredient:baclofen
Active Ingredient UNII:H789N3FKE8
Drugbank ID:DB00181
PubChem Compound:2284
CAS Number:1134-47-0
Dosage Form(s):injection
Route(s) Of Administrator:intrathecal
Daily Dose:
- 50.0 mg/day M03BX01
Chemical Structure: 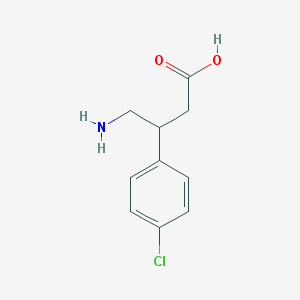

SMILE Code:
C1=CC(=CC=C1C(CC(=O)O)CN)Cl
C1=CC(=CC=C1C(CC(=O)O)CN)Cl
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Status dystonicus in childhood.
[Touati Nahla,Ben Rhouma Hanène,Kraoua Ichraf,Klaa Hédia,Turki Ilhem,Gouider-Khouja Neziha]Tunis Med.2015 Dec;93(12):756-9. PMID: 27249384
2: Movement disorder emergencies in childhood.
[Kirkham F J,Haywood P,Kashyape P,Borbone J,Lording A,Pryde K,Cox M,Keslake J,Smith M,Cuthbertson L,Murugan V,Mackie S,Thomas N H,Whitney A,Forrest K M,Parker A,Forsyth R,Kipps C M]Eur J Paediatr Neurol.2011 Sep;15(5):390-404. doi: 10.1016/j.ejpn.2011.04.005. Epub 2011 Aug 10. PMID: 21835657
3: Status dystonicus resembling the intrathecal baclofen withdrawal syndrome: a case report and review of the literature.
[Muirhead William,Jalloh Ibrahim,Vloeberghs Michael]J Med Case Rep.2010 Aug 31;4:294. doi: 10.1186/1752-1947-4-294. PMID: 20807402
4: Neuroleptic malignant syndrome from central nervous system insult: 4 cases and a novel treatment strategy. Clinical article.
[Wait Scott D,Ponce Francisco A,Killory Brendan D,Wallace Donna,Rekate Harold L]J Neurosurg Pediatr.2009 Sep;4(3):217-21. doi: 10.3171/2009.4.PEDS08444. PMID: 19772404
5: Intrathecal baclofen withdrawal resembling serotonin syndrome in an adolescent boy with cerebral palsy.
[Salazar Maria L,Eiland Lea S]Pediatr Emerg Care.2008 Oct;24(10):691-3. doi: 10.1097/PEC.0b013e318188a952. PMID: 19240673
6: An unusual presentation of baclofen overdose.
[Chong C-F,Wang T-L]Emerg Med J.2005 Sep;22(9):673-4. PMID: 16113202
7: Intrathecal baclofen withdrawal syndrome- a life-threatening complication of baclofen pump: a case report.
[Mohammed Imran,Hussain Asif]BMC Clin Pharmacol.2004 Aug 9;4:6. PMID: 15301690
8: Drug-induced rhabdomyolysis.
[Coco Teresa J,Klasner Ann E]Curr Opin Pediatr.2004 Apr;16(2):206-10. PMID: 15021204
9: Subdural catheter migration may lead to baclofen pump dysfunction.
[Pasquier Y,Cahana A,Schnider A]Spinal Cord.2003 Dec;41(12):700-2. PMID: 14639451
10: Monitoring of creatinine kinase during weaning of intrathecal baclofen and with symptoms of early withdrawal.
[Colachis Sam C,Rea Gary L]Am J Phys Med Rehabil.2003 Jun;82(6):489-92. PMID: 12820794
11: Cyproheptadine for intrathecal baclofen withdrawal.
[Meythaler Jay M,Roper James F,Brunner Robert C]Arch Phys Med Rehabil.2003 May;84(5):638-42. PMID: 12736874
12: Abrupt withdrawal from intrathecal baclofen: recognition and management of a potentially life-threatening syndrome.
[Coffey Robert J,Edgar Terence S,Francisco Gerard E,Graziani Virginia,Meythaler Jay M,Ridgely Patrick M,Sadiq Saud A,Turner Michael S]Arch Phys Med Rehabil.2002 Jun;83(6):735-41. PMID: 12048649
13: Intrathecal baclofen withdrawal simulating neuroleptic malignant syndrome in a child with cerebral palsy.
[Samson-Fang L,Gooch J,Norlin C]Dev Med Child Neurol.2000 Aug;42(8):561-5. PMID: 10981935
14: Status dystonicus: the syndrome and its management.
[Manji H,Howard R S,Miller D H,Hirsch N P,Carr L,Bhatia K,Quinn N,Marsden C D,Bahtia K]Brain.1998 Feb;121 ( Pt 2):243-52. PMID: 9549503
15: Hyperthermia, rhabdomyolysis, and disseminated intravascular coagulation associated with baclofen pump catheter failure.
[Reeves R K,Stolp-Smith K A,Christopherson M W]Arch Phys Med Rehabil.1998 Mar;79(3):353-6. PMID: 9523793
16: Severe dystonia and myoglobinuria.
[Jankovic J,Penn A S]Neurology.1982 Oct;32(10):1195-7. PMID: 6889706
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.