Search for drugs:
Typing the drug name to query
SIMVASTATIN
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacokinetics
- Simvastatin is a lactone that is readily hydrolyzed in vivo to the corresponding β-hydroxyacid, a potent inhibitor of HMG-CoA reductase. Inhibition of HMG-CoA reductase is the basis for an assay in pharmacokinetic studies of the β-hydroxyacid metabolites (active inhibitors) and, following base hydrolysis, active plus latent inhibitors (total inhibitors) in plasma following administration of simvastatin.
- The pharmacokinetics of simvastatin and simvastatin acid, following administration of the 80 mg Simvastatin Orally Disintegrating tablet and 240 mL water at 1 minute post-dosing, were comparable to those following administration of the simvastatin immediate release 80 mg tablet taken with 240 mL water.
- Following an oral dose of 14C-labeled simvastatin in man, 13% of the dose was excreted in urine and 60% in feces. Plasma concentrations of total radioactivity (simvastatin plus 14C-metabolites) peaked at 4 hours and declined rapidly to about 10% of peak by 12 hours postdose. Since simvastatin undergoes extensive first-pass extraction in the liver, the availability of the drug to the general circulation is low (<5%).
- Both simvastatin and its β-hydroxyacid metabolite are highly bound (approximately 95%) to human plasma proteins. Rat studies indicate that when radiolabeled simvastatin was administered, simvastatin-derived radioactivity crossed the blood-brain barrier.
- The major active metabolites of simvastatin present in human plasma are the β-hydroxyacid of simvastatin and its 6′-hydroxy, 6′-hydroxymethyl, and 6′-exomethylene derivatives. Peak plasma concentrations of both active and total inhibitors were attained within 1.3 to 2.4 hours postdose. While the recommended therapeutic dose range is 5 to 80 mg/day, there was no substantial deviation from linearity of AUC of inhibitors in the general circulation with an increase in dose to as high as 120 mg. Relative to the fasting state, the plasma profile of inhibitors was not affected when simvastatin was administered immediately before an American Heart Association recommended low-fat meal.
- In a study including 16 elderly patients between 70 and 78 years of age who received simvastatin 40 mg/day, the mean plasma level of HMG-CoA reductase inhibitory activity was increased approximately 45% compared with 18 patients between 18-30 years of age. Clinical study experience in the elderly (n=1522), suggests that there were no overall differences in safety between elderly and younger patients (see PRECAUTIONS, GERIATRIC USE).
- Kinetic studies with another reductase inhibitor, having a similar principal route of elimination, have suggested that for a given dose level higher systemic exposure may be achieved in patients with severe renal insufficiency (as measured by creatinine clearance).
- In a study of 12 healthy volunteers, simvastatin at the 80-mg dose had no effect on the metabolism of the probe cytochrome P450 isoform 3A4 (CYP3A4) substrates midazolam and erythromycin. This indicates that simvastatin is not an inhibitor of CYP3A4, and, therefore, is not expected to affect the plasma levels of other drugs metabolized by CYP3A4.
- Although the mechanism is not fully understood, cyclosporine has been shown to increase the AUC of HMG-CoA reductase inhibitors. The increase in AUC for simvastatin acid is presumably due, in part, to inhibition of CYP3A4.
- The risk of myopathy is increased by high levels of HMG-CoA reductase inhibitory activity in plasma. Potent inhibitors of CYP3A4 can raise the plasma levels of HMG-CoA reductase inhibitory activity and increase the risk of myopathy (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS and PRECAUTIONS, DRUG INTERACTIONS).
- Gemfibrozil: Coadministration of gemfibrozil (600 mg twice daily for 3 days) with simvastatin (40 mg daily) resulted in clinically significant increases in simvastatin acid AUC (185%) and Cmax (112%), possibly due to inhibition of simvastatin acid glucuronidation by gemfibrozil (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS, PRECAUTIONS, DRUG INTERACTIONS, DOSAGE AND ADMINISTRATION).
- Fenofibrate: Coadministration of fenofibrate (160 mg daily) with simvastatin (80 mg daily) for 7 days had no effect on plasma AUC (and Cmax) of either total HMG-CoA reductase inhibitory activity or fenofibric acid; there was a modest reduction (approximately 35%) of simvastatin acid which was not considered clinically significant (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS, PRECAUTIONS, DRUG INTERACTIONS).
- Simvastatin is a substrate for CYP3A4 (see PRECAUTIONS, DRUG INTERACTIONS). Grapefruit juice contains one or more components that inhibit CYP3A4 and can increase the plasma concentrations of drugs metabolized by CYP3A4. In one study1, 10 subjects consumed 200 mL of double-strength grapefruit juice (one can of frozen concentrate diluted with one rather than 3 cans of water) three times daily for 2 days and an additional 200 mL double-strength grapefruit juice together with, and 30 and 90 minutes following, a single dose of 60 mg simvastatin on the third day. This regimen of grapefruit juice resulted in mean increases in the concentration (as measured by the area under the concentration-time curve) of active and total HMG-CoA reductase inhibitory activity [measured using a radioenzyme inhibition assay both before (for active inhibitors) and after (for total inhibitors) base hydrolysis] of 2.4-fold and 3.6-fold, respectively, and of simvastatin and its β-hydroxyacid metabolite [measured using a chemical assay — liquid chromatography/tandem mass spectrometry] of 16-fold and 7-fold, respectively. In a second study, 16 subjects consumed one 8 oz glass of single-strength grapefruit juice (one can of frozen concentrate diluted with 3 cans of water) with breakfast for 3 consecutive days and a single dose of 20 mg simvastatin in the evening of the third day. This regimen of grapefruit juice resulted in a mean increase in the plasma concentration (as measured by the area under the concentration-time curve) of active and total HMG-CoA reductase inhibitory activity [using a validated enzyme inhibition assay different from that used in the first1 study, both before (for active inhibitors) and after (for total inhibitors) base hydrolysis] of 1.13-fold and 1.18-fold, respectively, and of simvastatin and its β-hydroxyacid metabolite [measured using a chemical assay — liquid chromatography/tandem mass spectrometry] of 1.88-fold and 1.31-fold, respectively. The effect of amounts of grapefruit juice between those used in these two studies on simvastatin pharmacokinetics has not been studied.
- WARNINGS
- Myopathy/Rhabdomyolysis
- Simvastatin, like other inhibitors of HMG-CoA reductase, occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by high levels of HMG-CoA reductase inhibitory activity in plasma.
- As with other HMG-CoA reductase inhibitors, the risk of myopathy/rhabdomyolysis is dose related. In a clinical trial database in which 41,050 patients were treated with simvastatin with 24,747 (approximately 60%) treated for at least 4 years, the incidence of myopathy was approximately 0.02%, 0.08% and 0.53% at 20, 40 and 80 mg/day, respectively. In these trials, patients were carefully monitored and some interacting medicinal products were excluded.
- All patients starting therapy with simvastatin or whose dose of simvastatin is being increased, should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness. Simvastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. Periodic CK determinations may be considered in patients starting therapy with simvastatin or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.
- Many of the patients who have developed rhabdomyolysis on therapy with simvastatin have had complicated medical histories, including renal insufficiency usually as a consequence of long-standing diabetes mellitus. Such patients merit closer monitoring. Therapy with simvastatin should be temporarily stopped a few days prior to elective major surgery and when any major medical or surgical condition supervenes.
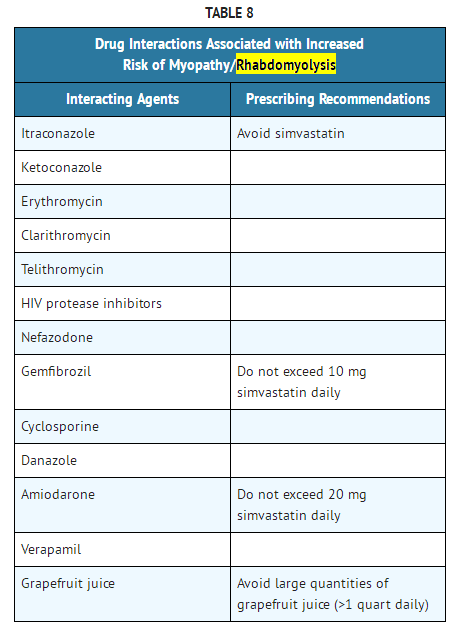
- The risk of myopathy/rhabdomyolysis is increased by concomitant use of simvastatin with the following:
- Potent inhibitors of CYP3A4: Simvastatin, like several other inhibitors of HMG-CoA reductase, is a substrate of cytochrome P450 3A4 (CYP3A4). When simvastatin is used with a potent inhibitor of CYP3A4, elevated plasma levels of HMG-CoA reductase inhibitory activity can increase the risk of myopathy and rhabdomyolysis, particularly with higher doses of simvastatin.
- Prescribing recommendations for interacting agents are summarized in Table 8
- PRECAUTIONS
- Information for Patients
- Patients should be advised about substances they should not take concomitantly with simvastatin and be advised to report promptly unexplained muscle pain, tenderness, or weakness (see LIST below and WARNINGS, MYOPATHY/RHABDOMYOLYSIS). Patients should also be advised to inform other physicians prescribing a new medication that they are taking Simvastatin Orally Disintegrating Tablets.
- Drug Interactions
- CYP3A4 Interactions
- Simvastatin is metabolized by CYP3A4 but has no CYP3A4 inhibitory activity; therefore it is not expected to affect the plasma concentrations of other drugs metabolized by CYP3A4. Potent inhibitors of CYP3A4 (below) increase the risk of myopathy by reducing the elimination of simvastatin.
- See WARNINGS, MYOPATHY/RHABDOMYOLYSIS, and CLINICAL PHARMACOLOGY, PHARMACOKINETICS.
- Interactions with lipid-lowering drugs that can cause myopathy when given alone
- See WARNINGS, MYOPATHY/RHABDOMYOLYSIS.
- The risk of myopathy is increased by gemfibrozil (see DOSAGE AND ADMINISTRATION) and to a lesser extent by other fibrates and niacin (nicotinic acid) (≥1 g/day).
- Other drug interactions
- Cyclosporine or Danazol
- The risk of myopathy/rhabdomyolysis is increased by concomitant administration of cyclosporine or danazol particularly with higher doses of simvastatin (see CLINICAL PHARMACOLOGY, PHARMACOKINETICS; WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- Amiodarone or Verapamil
- The risk of myopathy/rhabdomyolysis is increased by concomitant administration of amiodarone or verapamil with higher doses of simvastatin (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- Geriatric Use
- A pharmacokinetic study with simvastatin showed the mean plasma level of HMG-CoA reductase inhibitory activity to be approximately 45% higher in elderly patients between 70-78 years of age compared with patients between 18-30 years of age. In 4S, 1,021 (23%) of 4,444 patients were 65 or older. In 4S, lipid-lowering efficacy was at least as great in elderly patients compared with younger patients. In this study, simvastatin significantly reduced total mortality and CHD mortality in elderly patients with a history of CHD. In HPS, 52% of patients were elderly (4,891 patients 65-69 years and 5,806 patients 70 years or older). The relative risk reductions of CHD death, non-fatal MI, coronary and non-coronary revascularization procedures, and stroke were similar in older and younger patients (see CLINICAL PHARMACOLOGY). In HPS, among 32,145 patients entering the active run-in period, there were 2 cases of myopathy/rhabdomyolysis; these patients were aged 67 and 73. Of the 7 cases of myopathy/rhabdomyolysis among 10,269 patients allocated to simvastatin, 4 were aged 65 or more (at baseline), of whom one was over 75. There were no overall differences in safety between older and younger patients in either 4S or HPS.
- ADVERSE REACTIONS
- Heart Protection Study
- Clinical Adverse Experiences
- In HPS (see CLINICAL PHARMACOLOGY, CLINICAL STUDIES), involving 20,536 patients treated with simvastatin 40 mg/day (n=10,269) or placebo (n=10,267), the safety profiles were comparable between patients treated with simvastatin and patients treated with placebo over the mean 5 years of the study. In this large trial, only serious adverse events and discontinuations due to any adverse events were recorded. Discontinuation rates due to adverse experiences were comparable (4.8% in patients treated with simvastatin compared with 5.1% in patients treated with placebo). The incidence of myopathy/rhabdomyolysis was <0.1% in patients treated with simvastatin.
- The following effects have been reported with drugs in this class. Not all the effects listed below have necessarily been associated with simvastatin therapy.
- Skeletal: muscle cramps, myalgia, myopathy, rhabdomyolysis, arthralgias.
- Neurological: dysfunction of certain cranial nerves (including alteration of taste, impairment of extra-ocular movement, facial paresis), tremor, dizziness, vertigo, memory loss, paresthesia, peripheral neuropathy, peripheral nerve palsy, psychic disturbances, anxiety, insomnia, depression.
- Hypersensitivity Reactions: An apparent hypersensitivity syndrome has been reported rarely which has included one or more of the following features: anaphylaxis, angioedema, lupus erythematous-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia, arthritis, arthralgia, urticaria, asthenia, photosensitivity, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
- Gastrointestinal: pancreatitis, hepatitis, including chronic active hepatitis, cholestatic jaundice, fatty change in liver, and, rarely, cirrhosis, fulminant hepatic necrosis, and hepatoma; anorexia, vomiting.
- Skin: alopecia, pruritus. A variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails) have been reported.
- Reproductive: gynecomastia, loss of libido, erectile dysfunction.
- Eye: progression of cataracts (lens opacities), ophthalmoplegia.
- Laboratory Abnormalities: elevated transaminases, alkaline phosphatase, γ-glutamyl transpeptidase, and bilirubin; thyroid function abnormalities.
- Laboratory Tests
- Marked persistent increases of serum transaminases have been noted (see WARNINGS, LIVER DYSFUNCTION). About 5% of patients had elevations of CK levels of 3 or more times the normal value on one or more occasions. This was attributable to the noncardiac fraction of CK. Muscle pain or dysfunction usually was not reported (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- Concomitant Lipid-Lowering Therapy
- In controlled clinical studies in which simvastatin was administered concomitantly with cholestyramine, no adverse reactions peculiar to this concomitant treatment were observed. The adverse reactions that occurred were limited to those reported previously with simvastatin or cholestyramine. The combined use of simvastatin at doses exceeding 10 mg/day with gemfibrozil should be avoided (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- DOSAGE AND ADMINISTRATION
- Concomitant Lipid-Lowering Therapy
- Simvastatin Orally Disintegrating Tablets are effective alone or when used concomitantly with bile-acid sequestrants. If Simvastatin Orally Disintegrating Tablets are used in combination with gemfibrozil, the dose of Simvastatin Orally Disintegrating Tablets should not exceed 10 mg/day (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS and PRECAUTIONS, DRUG INTERACTIONS).
- Patients taking Cyclosporine or Danazol
- In patients taking cyclosporine or danazol concomitantly with Simvastatin Orally Disintegrating Tablets (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS), therapy should begin with 5 mg/day and should not exceed 10 mg/day. Simvastatin Orally Disintegrating Tablets are not available in the 5 mg dosage strength. Other simvastatin 5 mg tablets should be used if a 5 mg dose is needed.
- Patients taking Amiodarone or Verapamil
- In patients taking amiodarone or verapamil concomitantly with Simvastatin Orally Disintegrating Tablets, the dose should not exceed 20 mg/day (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS and PRECAUTIONS, DRUG INTERACTIONS, OTHER DRUG INTERACTIONS).
- Patients with Renal Insufficiency
- Because Simvastatin Orally Disintegrating Tablets do not undergo significant renal excretion, modification of dosage should not be necessary in patients with mild to moderate renal insufficiency. However, caution should be exercised when Simvastatin Orally Disintegrating Tablets are administered to patients with severe renal insufficiency; such patients should be started at 5 mg/day and be closely monitored (see CLINICAL PHARMACOLOGY, PHARMACOKINETICS and WARNINGS, MYOPATHY/RHABDOMYOLYSIS). Simvastatin Orally Disintegrating Tablets are not available in the 5 mg dosage strength. Other simvastatin 5 mg tablets should be used if a 5 mg dose is needed.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
5721
37191
Other ADRs
32522
14084757
Odds Ratio = 66.621
Drug Property Information
ATC Code(s):
- C10BX04 - simvastatin
- C10BX - "HMG CoA reductase inhibitors, other combinations"
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BA02 - simvastatin
- C10BA - HMG CoA reductase inhibitors in combination with other lipid modifying agents
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BA04 - simvastatin
- C10BA - HMG CoA reductase inhibitors in combination with other lipid modifying agents
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- A10BH51 - simvastatin
- A10BH - Dipeptidyl peptidase 4 (DPP-4) inhibitors
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
- C10AA01 - simvastatin
- C10AA - HMG CoA reductase inhibitors
- C10A - "LIPID MODIFYING AGENTS, PLAIN"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BX01 - simvastatin
- C10BX - "HMG CoA reductase inhibitors, other combinations"
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:simvastatin
Active Ingredient UNII:AGG2FN16EV
Drugbank ID:DB00641
PubChem Compound:54454
CAS Number:79902-63-9
Dosage Form(s):tablet, orally disintegrating
Route(s) Of Administrator:oral
Daily Dose:
- 30.0 mg/day C10AA01
Chemical Structure: 

SMILE Code:
CCC(C)(C)C(=O)O[C@H]1C[C@H](C=C2[C@H]1[C@H]([C@H](C=C2)C)CC[C@@H]3C[C@H](CC(=O)O3)O)C
CCC(C)(C)C(=O)O[C@H]1C[C@H](C=C2[C@H]1[C@H]([C@H](C=C2)C)CC[C@@H]3C[C@H](CC(=O)O3)O)C
Reference
COHORT STUDY:
1: HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment.
[HPS2-THRIVE Collaborative Group, Eur Heart J. 2013 May;34(17):1279-91.]ABSTRACT
AIMS: Niacin has potentially favourable effects on lipids, but its effect on cardiovascular outcomes is uncertain. HPS2-THRIVE is a large randomized trial assessing the effects of extended release (ER) niacin in patients at high risk of vascular events.
METHODS AND RESULTS: Prior to randomization, 42 424 patients with occlusive arterial disease were given simvastatin 40 mg plus, if required, ezetimibe 10 mg daily to standardize their low-density lipoprotein (LDL)-lowering therapy. The ability to remain compliant with ER niacin 2 g plus laropiprant 40 mg daily (ERN/LRPT) for ~1 month was then assessed in 38 369 patients and about one-third were excluded (mainly due to niacin side effects). A total of 25 673 patients were randomized between ERN/LRPT daily vs. placebo and were followed for a median of 3.9 years. By the end of the study, 25% of participants allocated ERN/LRPT vs. 17% allocated placebo had stopped their study treatment. The most common medical reasons for stopping ERN/LRPT were related to skin, gastrointestinal, diabetes, and musculoskeletal side effects. When added to statin-based LDL-lowering therapy, allocation to ERN/LRPT increased the risk of definite myopathy [75 (0.16%/year) vs. 17 (0.04%/year): risk ratio 4.4; 95% CI 2.6-7.5; P < 0.0001]; 7 vs. 5 were rhabdomyolysis. Any myopathy (definite or incipient) was more common among participants in China [138 (0.66%/year) vs. 27 (0.13%/year)] than among those in Europe [17 (0.07%/year) vs. 11 (0.04%/year)]. Consecutive alanine transaminase >3× upper limit of normal, in the absence of muscle damage, was seen in 48 (0.10%/year) ERN/LRPT vs. 30 (0.06%/year) placebo allocated participants.
CONCLUSION: The risk of myopathy was increased by adding ERN/LRPT to simvastatin 40 mg daily (with or without ezetimibe), particularly in Chinese patients whose myopathy rates on simvastatin were higher. Despite the side effects of ERN/LRPT, among individuals who were able to tolerate it for ~1 month, three-quarters continued to take it for ~4 years.
PMID: 23444397
METHODS AND RESULTS: Prior to randomization, 42 424 patients with occlusive arterial disease were given simvastatin 40 mg plus, if required, ezetimibe 10 mg daily to standardize their low-density lipoprotein (LDL)-lowering therapy. The ability to remain compliant with ER niacin 2 g plus laropiprant 40 mg daily (ERN/LRPT) for ~1 month was then assessed in 38 369 patients and about one-third were excluded (mainly due to niacin side effects). A total of 25 673 patients were randomized between ERN/LRPT daily vs. placebo and were followed for a median of 3.9 years. By the end of the study, 25% of participants allocated ERN/LRPT vs. 17% allocated placebo had stopped their study treatment. The most common medical reasons for stopping ERN/LRPT were related to skin, gastrointestinal, diabetes, and musculoskeletal side effects. When added to statin-based LDL-lowering therapy, allocation to ERN/LRPT increased the risk of definite myopathy [75 (0.16%/year) vs. 17 (0.04%/year): risk ratio 4.4; 95% CI 2.6-7.5; P < 0.0001]; 7 vs. 5 were rhabdomyolysis. Any myopathy (definite or incipient) was more common among participants in China [138 (0.66%/year) vs. 27 (0.13%/year)] than among those in Europe [17 (0.07%/year) vs. 11 (0.04%/year)]. Consecutive alanine transaminase >3× upper limit of normal, in the absence of muscle damage, was seen in 48 (0.10%/year) ERN/LRPT vs. 30 (0.06%/year) placebo allocated participants.
CONCLUSION: The risk of myopathy was increased by adding ERN/LRPT to simvastatin 40 mg daily (with or without ezetimibe), particularly in Chinese patients whose myopathy rates on simvastatin were higher. Despite the side effects of ERN/LRPT, among individuals who were able to tolerate it for ~1 month, three-quarters continued to take it for ~4 years.
OTHER REFERENCE(S):
1: Comparative impact of systemic delivery of atorvastatin, simvastatin, and lovastatin on bone mineral density of the ovariectomized rats.
[Shahrezaee Mostafa,Oryan Ahmad,Bastami Farshid,Hosseinpour Sepanta,Shahrezaee Mohammad Hossein,Kamali Amir]Endocrine.2018 Jan 25. doi: 10.1007/s12020-018-1531-6. [Epub ahead of print] PMID: 29372484
2: Evaluation of Trace Elements in Augmentation of Statin-Induced Cytotoxicity in Uremic Serum-Exposed Human Rhabdomyosarcoma Cells.
[Uchiyama Hitoshi,Tsujimoto Masayuki,Shimada Naomi,Tsutsui Koji,Nitta Ayaka,Yoshida Takuya,Furukubo Taku,Izumi Satoshi,Yamakawa Tomoyuki,Tachiki Hidehisa,Minegaki Tetsuya,Nishiguchi Kohshi]Toxins (Basel).2018 Jan 25;10(2). pii: E53. doi: 10.3390/toxins10020053. PMID: 29370118
3: Simvastatin activates single skeletal RyR1 channels but exerts more complex regulation of the cardiac RyR2 isoform.
[Venturi Elisa,Lindsay Chris,Lotteau Sabine,Yang Zhaokang,Steer Emma,Witschas Katja,Wilson Abigail D,Wickens James R,Russell Angela J,Steele Derek,Calaghan Sarah,Sitsapesan Rebecca]Br J Pharmacol.2018 Mar;175(6):938-952. doi: 10.1111/bph.14136. Epub 2018 Feb 5. PMID: 29278865
4: Muscle Damage Due to Fusidic Acid-Statin Interaction: Review of 75 Cases From the French Pharmacovigilance Database and Literature Reports.
[Bataillard Maxime,Beyens Marie-Noëlle,Mounier Geneviève,Vergnon-Miszczycha Delphine,Bagheri Haleh,Cathebras Pascal]Am J Ther.2017 Nov 9. doi: 10.1097/MJT.0000000000000679. [Epub ahead of print] PMID: 29189310
5: Ticagrelor Leads to Statin-Induced Rhabdomyolysis: A Case Report.
[Mrotzek Simone M,Rassaf Tienush,Totzeck Matthias]Am J Case Rep.2017 Nov 23;18:1238-1241. PMID: 29167415
6: Simvastatin dose and acute kidney injury without concurrent serious muscle injury: A nationwide nested case-control study.
[Parkin Lianne,Sharples Katrina J,Barson David J,Blank Mei-Ling]PLoS One.2017 Jul 28;12(7):e0182066. doi: 10.1371/journal.pone.0182066. eCollection 2017. PMID: 28753656
7: Bone scintigraphy of severe hypercalcemia following simvastatin induced rhabdomyolysis.
[Mirza Zubair B,Hu Sophia,Amorosa Louis F]Clin Cases Miner Bone Metab.2016 Sep-Dec;13(3):257-261. doi: 10.11138/ccmbm/2016.13.3.257. Epub 2017 Feb 10. PMID: 28228795
8: Colchicine triggered severe rhabdomyolysis after long-term low-dose simvastatin therapy: a case report.
[Frydrychowicz Clara,Pasieka Bastian,Pierer Matthias,Mueller Wolf,Petros Sirak,Weidhase Lorenz]J Med Case Rep.2017 Jan 4;11(1):8. doi: 10.1186/s13256-016-1169-z. PMID: 28049514
9: Low-dose Simvastatin Increases Skeletal Muscle Sensitivity to Caffeine and Halothane.
[Cui Xu-Lei,Wang Ying-Lin,Tan Gang,Luo Ai-Lun,Guo Xiang-Yang]Chin Med Sci J.2016 Jun 20;31(2):107-115. PMID: 28031099
10: [Medication management: Simvastatin and Amlodipin - a clinically relevant drug-interaction?]
[Schröder Jane,Goltz Lisa,Knoth Holger]Dtsch Med Wochenschr.2016 Oct;141(21):1575-1577. Epub 2016 Oct 17. PMID: 27750346
11: [Myopathy in a patient during simvastatin and fluconazole treatment].
[Pedersen Jens Kristian,Lydolph Magnus Christian,Somnier Finn,Junker Peter]Ugeskr Laeger.2016 Sep 26;178(39). pii: V04160257. PMID: 27697125
12: Current statins show calcium channel blocking activity through voltage gated channels.
[Ali Niaz,Begum Robina,Faisal Muhammad Saleh,Khan Aslam,Nabi Muhammad,Shehzadi Gulfam,Ullah Shakir,Ali Waqar]BMC Pharmacol Toxicol.2016 Sep 21;17(1):43. doi: 10.1186/s40360-016-0086-5. PMID: 27649899
13: Gluteal rhabdomyolysis and renal failure due to simvastatin and amiodarone.
[Semmo Mariam,Dhayat Nasser Abdalla]Kidney Int.2016 Oct;90(4):909. doi: 10.1016/j.kint.2016.06.025. PMID: 27633874
14: Interpretation of the evidence for the efficacy and safety of statin therapy.
[Collins Rory,Reith Christina,Emberson Jonathan,Armitage Jane,Baigent Colin,Blackwell Lisa,Blumenthal Roger,Danesh John,Smith George Davey,DeMets David,Evans Stephen,Law Malcolm,MacMahon Stephen,Martin Seth,Neal Bruce,Poulter Neil,Preiss David,Ridker Paul,Roberts Ian,Rodgers Anthony,Sandercock Peter,Schulz Kenneth,Sever Peter,Simes John,Smeeth Liam,Wald Nicholas,Yusuf Salim,Peto Richard]Lancet.2016 Nov 19;388(10059):2532-2561. doi: 10.1016/S0140-6736(16)31357-5. Epub 2016 Sep 8. PMID: 27616593
15: Statin-associated rhabdomyolysis triggered by drug-drug interaction with itraconazole.
[Dybro Anne Mette,Damkier Per,Rasmussen Torsten Bloch,Hellfritzsch Maja]BMJ Case Rep.2016 Sep 7;2016. pii: bcr2016216457. doi: 10.1136/bcr-2016-216457. PMID: 27605198
16: The Risk of Hepatotoxicity, New Onset Diabetes and Rhabdomyolysis in the Era of High-Intensity Statin Therapy: Does Statin Type Matter?
[Benes Lane B,Bassi Nikhil S,Davidson Michael H]Prog Cardiovasc Dis.2016 Sep - Oct;59(2):145-152. doi: 10.1016/j.pcad.2016.08.001. Epub 2016 Aug 5. PMID: 27503844
17: Ciprofloxacin and statin interaction: a cautionary tale of rhabdomyolysis.
[Goldie Fraser Charles,Brogan Amy,Boyle James Graham]BMJ Case Rep.2016 Jul 28;2016. pii: bcr2016216048. doi: 10.1136/bcr-2016-216048. PMID: 27469384
18: A benefit-risk assessment model for statins using multicriteria decision analysis based on a discrete choice experiment in Korean patients.
[Byun Ji-Hye,Kwon Sun-Hong,Ha Ji-Hye,Lee Eui-Kyung]Ther Clin Risk Manag.2016 Jun 13;12:965-74. doi: 10.2147/TCRM.S100438. eCollection 2016. PMID: 27358567
19: [Weakness of the extremities in a 73-year-old male patient].
[Attaran-Bandarabadi M,Kalbasi Anaraki P,Gwinner W,Haller H]Internist (Berl).2016 Aug;57(8):815-8. doi: 10.1007/s00108-016-0060-8. PMID: 27167632
20: [Brown urine : Myoglobin-induced renal failure after concomitant administration of simvastatin and amiodarone].
[Pietsch U,Müller-Höcker C,Filipovic M]Anaesthesist.2016 May;65(5):366-8. doi: 10.1007/s00101-016-0171-6. Epub 2016 May 3. PMID: 27142363
21: The Antimicrobial Agent Fusidic Acid Inhibits Organic Anion Transporting Polypeptide-Mediated Hepatic Clearance and May Potentiate Statin-Induced Myopathy.
[Eng Heather,Scialis Renato J,Rotter Charles J,Lin Jian,Lazzaro Sarah,Varma Manthena V,Di Li,Feng Bo,West Michael,Kalgutkar Amit S]Drug Metab Dispos.2016 May;44(5):692-9. doi: 10.1124/dmd.115.067447. Epub 2016 Feb 17. PMID: 26888941
22: Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis.
[Abraldes Juan G,Villanueva Candid,Aracil Carles,Turnes Juan,Hernandez-Guerra Manuel,Genesca Joan,Rodriguez Manuel,Castellote Jose,García-Pagán Juan Carlos,Torres Ferran,Calleja Jose Luis,Albillos Agustin,Bosch Jaime,BLEPS Study Group]Gastroenterology.2016 May;150(5):1160-1170.e3. doi: 10.1053/j.gastro.2016.01.004. Epub 2016 Jan 14. PMID: 26774179
23: Pharmacokinetic model for the inhibition of simvastatin metabolism by itraconazole.
[Lohitnavy Manupat,Methaneethorn Janthima,Chiang-Ngernthanyakool Rangsimaporn,Tongpeng Wasinee,Chan-Im Daranee,Phaohorm Suttipong]Conf Proc IEEE Eng Med Biol Soc.2015;2015:3246-9. doi: 10.1109/EMBC.2015.7319084. PMID: 26736984
24: Association Between SLCO1B1 Gene T521C Polymorphism and Statin-Related Myopathy Risk: A Meta-Analysis of Case-Control Studies.
[Hou Qingtao,Li Sheyu,Li Ling,Li Yun,Sun Xin,Tian Haoming]Medicine (Baltimore).2015 Sep;94(37):e1268. doi: 10.1097/MD.0000000000001268. PMID: 26376374
25: Grapefruit Juice and Statins.
[Lee Jonathan W,Morris Joan K,Wald Nicholas J]Am J Med.2016 Jan;129(1):26-9. doi: 10.1016/j.amjmed.2015.07.036. Epub 2015 Aug 20. PMID: 26299317
26: Safety considerations with fenofibrate/simvastatin combination.
[Filippatos Theodosios D,Elisaf Moses S]Expert Opin Drug Saf.2015;14(9):1481-93. doi: 10.1517/14740338.2015.1056778. Epub 2015 Jul 3. PMID: 26134595
27: From a fish tank injury to hospital haemodialysis: the serious consequences of drug interactions.
[Hill Fay Joanne,McCloskey Sarah Jane,Sheerin Neil]BMJ Case Rep.2015 Jun 23;2015. pii: bcr2015209961. doi: 10.1136/bcr-2015-209961. PMID: 26106178
28: Drug-drug interactions that interfere with statin metabolism.
[Hirota Takeshi,Ieiri Ichiro]Expert Opin Drug Metab Toxicol.2015;11(9):1435-47. doi: 10.1517/17425255.2015.1056149. Epub 2015 Jun 11. PMID: 26058399
29: Adverse effects of low-dose systemic cyclosporine therapy in high-risk penetrating keratoplasty.
[Lee Jong Joo,Kim Mee Kum,Wee Won Ryang]Graefes Arch Clin Exp Ophthalmol.2015 Jul;253(7):1111-9. doi: 10.1007/s00417-015-3008-0. Epub 2015 Apr 21. PMID: 25896110
30: Severe rhabdomyolysis associated with simvastatin and role of ciprofloxacin and amlodipine coadministration.
[De Schryver Nicolas,Wittebole Xavier,Van den Bergh Peter,Haufroid Vincent,Goffin Eric,Hantson Philippe]Case Rep Nephrol.2015;2015:761393. doi: 10.1155/2015/761393. Epub 2015 Mar 26. PMID: 25883814
31: [Renoprotective effects of statins under the conditions of acute renal failure, caused by rhabdomyolysis].
[Zamorskiĭ I I,Zeleniuk V G]Biofizika.2014 Sep-Oct;59(5):1027-30. PMID: 25730990
32: Sitagliptin/Simvastatin: a first combination tablet to treat type 2 diabetes and hypercholesterolemia--a review of its characteristics.
[Ramadan Wijdan H,Kabbara Wissam K]Vasc Health Risk Manag.2015 Feb 11;11:125-32. doi: 10.2147/VHRM.S79198. eCollection 2015. PMID: 25709467
33: Risk identification and possible countermeasures for muscle adverse effects during statin therapy.
[Magni Paolo,Macchi Chiara,Morlotti Beatrice,Sirtori Cesare R,Ruscica Massimiliano]Eur J Intern Med.2015 Mar;26(2):82-8. doi: 10.1016/j.ejim.2015.01.002. Epub 2015 Jan 29. PMID: 25640999
34: [Clinical analysis of 160 cases of statin-induced myopathy].
[Jiang Yuexin,Lou Ying,Liu Yuqing,Wang Li,Pang Huimin,Zhang Jun,Zhou Yingqun,Li Yishi]Zhonghua Xin Xue Guan Bing Za Zhi.2014 Nov;42(11):905-9. PMID: 25620251
35: Inhibition of prenyltransferase activity by statins in both liver and muscle cell lines is not causative of cytotoxicity.
[Gee Rowena H,Spinks Jenny N,Malia Jason M,Johnston Jonathan D,Plant Nick J,Plant Kathryn E]Toxicology.2015 Mar 2;329:40-8. doi: 10.1016/j.tox.2015.01.005. Epub 2015 Jan 8. PMID: 25578243
36: Pharmacokinetic modeling of simvastatin, nelfinavir and their interaction in humans.
[Methaneethorn Janthima,Kunyamee Patcharaporn,Jindasri Warangkana,Wattanasaovaluk Warunee,Kraiboot Anoot,Lohitnavy Manupat]Conf Proc IEEE Eng Med Biol Soc.2014;2014:5715-8. doi: 10.1109/EMBC.2014.6944925. PMID: 25571293
37: A pharmacokinetic drug-drug interaction model of simvastatin and verapamil in humans.
[Methaneethorn Janthima,Chamnansua Munlikar,Kaewdang Natnaree,Lohitnavy Manupat]Conf Proc IEEE Eng Med Biol Soc.2014;2014:5711-4. doi: 10.1109/EMBC.2014.6944924. PMID: 25571292
38: A pharmacokinetic drug-drug interaction model of simvastatin and clarithromycin in humans.
[Methaneethorn Janthima,Chaiwong Krissanapong,Pongpanich Komwut,Sonsingh Phakawat,Lohitnavy Manupat]Conf Proc IEEE Eng Med Biol Soc.2014;2014:5703-6. doi: 10.1109/EMBC.2014.6944922. PMID: 25571290
39: [Myopathy and rhabdomyolysis after treatment with simvastatin, amlodipine, and roxithromycin].
[Skovbølling Sara Lyngby,Lindelof Mette]Ugeskr Laeger.2014 Oct 6;176(41). pii: V04140212. PMID: 25331664
40: GATM locus does not replicate in rhabdomyolysis study.
[Floyd James S,Bis Joshua C,Brody Jennifer A,Heckbert Susan R,Rice Kenneth,Psaty Bruce M]Nature.2014 Sep 18;513(7518):E1-3. doi: 10.1038/nature13629. PMID: 25230668
41: Risk of statin-induced rhabdomyolysis in patients with hepatic impairment.
[Kolhe Nitin,Lewis Jeremy,McCulloch Thomas Alasdair]BMJ Case Rep.2014 Sep 11;2014. pii: bcr2014204013. doi: 10.1136/bcr-2014-204013. PMID: 25213784
42: Uremic toxins enhance statin-induced cytotoxicity in differentiated human rhabdomyosarcoma cells.
[Uchiyama Hitoshi,Tsujimoto Masayuki,Shinmoto Tadakazu,Ogino Hitomi,Oda Tomoko,Yoshida Takuya,Furukubo Taku,Izumi Satoshi,Yamakawa Tomoyuki,Tachiki Hidehisa,Minegaki Tetsuya,Nishiguchi Kohshi]Toxins (Basel).2014 Sep 3;6(9):2612-25. doi: 10.3390/toxins6092612. PMID: 25192420
43: Acute rhabdomyolysis associated with coadministration of levofloxacin and simvastatin in a patient with normal renal function.
[Paparoupa Maria,Pietrzak Sebastian,Gillissen Adrian]Case Rep Med.2014;2014:562929. doi: 10.1155/2014/562929. Epub 2014 Jul 22. PMID: 25140181
44: Synthesis and characterization of cationic polymeric nanoparticles as simvastatin carriers for enhancing the osteogenesis of bone marrow mesenchymal stem cells.
[Wang Chau-Zen,Fu Yin-Chih,Jian Shih-Ciang,Wang Yan-Hsiung,Liu Po-Len,Ho Mei-Ling,Wang Chih-Kuang]J Colloid Interface Sci.2014 Oct 15;432:190-9. doi: 10.1016/j.jcis.2014.06.037. Epub 2014 Jun 22. PMID: 25086394
45: Rhabdomyolysis in association with simvastatin and dosage increment in clarithromycin.
[Page S R,Yee K C]Intern Med J.2014 Jul;44(7):690-3. doi: 10.1111/imj.12464. PMID: 25041770
46: Mortality from common drug interactions systems, knowledge and clinical reasoning to optimise prescribing.
[Martin J H,Coombes I]Intern Med J.2014 Jul;44(7):621-4. doi: 10.1111/imj.12473. PMID: 25041768
47: Non-cardiovascular effects associated with statins.
[Desai Chintan S,Martin Seth S,Blumenthal Roger S]BMJ.2014 Jul 17;349:g3743. doi: 10.1136/bmj.g3743. PMID: 25035309
48: [Renoprotective efficacy of different doses of statins in experimental acute renal failure].
[Zeleniuk V H,Zamors'kyĭ I I,Horoshko O M]Fiziol Zh.2014;60(2):75-81. PMID: 25007525
49: Rhabdomyolysis in a hepatitis C virus infected patient treated with telaprevir and simvastatin.
[Kanter Clara T M M de,Luin Matthijs van,Solas Caroline,Burger David M,Vrolijk Jan Maarten]Ann Hepatol.2014 Jul-Aug;13(4):452-5. PMID: 24927617
50: [Rhabdomyolysis and severe hepatotoxicity due to a drug-drug interaction between ritonavir and simvastatin. Could we use the most cost-effective statin in all human immunodeficiency virus-infected patients?].
[Bastida Carla,Also Maria Antonia,Pericas Juan Manuel,Letang Emili,Tuset Montse,Miró Josep Maria]Enferm Infecc Microbiol Clin.2014 Nov;32(9):579-82. doi: 10.1016/j.eimc.2014.03.014. Epub 2014 Jun 7. PMID: 24913991
51: Management of a mixed overdose of calcium channel blockers, β-blockers and statins.
[Thakrar Reena,Shulman Rob,Bellingan Geoff,Singer Mervyn]BMJ Case Rep.2014 Jun 6;2014. pii: bcr2014204732. doi: 10.1136/bcr-2014-204732. PMID: 24907219
52: Statin-induced rhabdomyolysis: a comprehensive review of case reports.
[Mendes Polyana,Robles Priscila Games,Mathur Sunita]Physiother Can.2014 Spring;66(2):124-32. doi: 10.3138/ptc.2012-65. PMID: 24799748
53: [Rhabdomyolysis secondary to simvastatin and phenofibrate].
[Forcadell-Peris M J,de Diego-Cabanes C]Semergen.2014 May-Jun;40(4):e91-4. doi: 10.1016/j.semerg.2014.01.007. Epub 2014 Apr 24. PMID: 24768027
54: Low vitamin D as a risk factor for the development of myalgia in patients taking high-dose simvastatin: a retrospective review.
[Mergenhagen Kari,Ott Michael,Heckman Kerry,Rubin Lisa M,Kellick Kenneth]Clin Ther.2014 May;36(5):770-7. doi: 10.1016/j.clinthera.2014.02.023. Epub 2014 Apr 16. PMID: 24742497
55: Simvastatin dose and risk of rhabdomyolysis: nested case-control study based on national health and drug dispensing data.
[Parkin Lianne,Paul Charlotte,Herbison G Peter]Int J Cardiol.2014 Jun 1;174(1):83-9. doi: 10.1016/j.ijcard.2014.03.150. Epub 2014 Mar 28. PMID: 24726164
56: Selective serotonin reuptake inhibitor drug interactions in patients receiving statins.
[Andrade Chittaranjan]J Clin Psychiatry.2014 Feb;75(2):e95-9. doi: 10.4088/JCP.13f08941. PMID: 24602259
57: Predictors and outcomes of increases in creatine phosphokinase concentrations or rhabdomyolysis risk during statin treatment.
[van Staa Tjeerd P,Carr Daniel F,O'Meara Helen,McCann Gerry,Pirmohamed Munir]Br J Clin Pharmacol.2014 Sep;78(3):649-59. doi: 10.1111/bcp.12367. PMID: 24602118
58: SLCO1B1 Polymorphisms and Statin-Induced Myopathy.
[Stewart Alison]PLoS Curr.2013 Dec 4;5. pii: ecurrents.eogt.d21e7f0c58463571bb0d9d3a19b82203. doi: 10.1371/currents.eogt.d21e7f0c58463571bb0d9d3a19b82203. PMID: 24459608
59: Neuropsychiatric adverse events associated with statins: epidemiology, pathophysiology, prevention and management.
[Tuccori Marco,Montagnani Sabrina,Mantarro Stefania,Capogrosso-Sansone Alice,Ruggiero Elisa,Saporiti Alessandra,Antonioli Luca,Fornai Matteo,Blandizzi Corrado]CNS Drugs.2014 Mar;28(3):249-72. doi: 10.1007/s40263-013-0135-1. PMID: 24435290
60: Lipid-lowering agents for nephrotic syndrome.
[Kong Xiangyu,Yuan Hao,Fan Junming,Li Zi,Wu Taixiang,Jiang Lanhui]Cochrane Database Syst Rev.2013 Dec 10;(12):CD005425. doi: 10.1002/14651858.CD005425.pub2. PMID: 24327265
61: Comparative efficacy and adverse effects of the addition of ezetimibe to statin versus statin titration in chronic kidney disease patients.
[Suzuki Hiromichi,Watanabe Yusuke,Kumagai Hiroo,Shuto Hiroshi]Ther Adv Cardiovasc Dis.2013 Dec;7(6):306-15. doi: 10.1177/1753944713513222. Epub 2013 Nov 26. PMID: 24280596
62: [Hypolipidemic agents drug interactions: approach to establish and assess its clinical significance. Structured review].
[Franco D,Henao Y,Monsalve M,Gutiérrez F,Hincapie J,Amariles P]Farm Hosp.2013 Nov-Dec;37(6):539-57. doi: 10.7399/FH.2013.37.6.1077. PMID: 24256019
63: Treatment of dyslipidemia in chronic kidney disease: Effectiveness and safety of statins.
[Scarpioni Roberto,Ricardi Marco,Albertazzi Vittorio,Melfa Luigi]World J Nephrol.2012 Dec 6;1(6):184-94. doi: 10.5527/wjn.v1.i6.184. PMID: 24175258
64: Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients.
[Hong Jung Yong,Nam Eun Mi,Lee Jeeyun,Park Joon Oh,Lee Sang-Cheol,Song Seo-Young,Choi Seong Ho,Heo Jin Seok,Park Se Hoon,Lim Ho Yeong,Kang Won Ki,Park Young Suk]Cancer Chemother Pharmacol.2014 Jan;73(1):125-30. doi: 10.1007/s00280-013-2328-1. Epub 2013 Oct 27. PMID: 24162380
65: Effect of simvastatin-amiodarone drug interaction alert on appropriate prescribing.
[Prom Rathasen,Umscheid Craig A,Kasbekar Nishaminy,Spinler Sarah A]Am J Health Syst Pharm.2013 Nov 1;70(21):1878-9. doi: 10.2146/ajhp120553. PMID: 24128959
66: SLCO1B1 genetic variant associated with statin-induced myopathy: a proof-of-concept study using the clinical practice research datalink.
[Carr D F,O'Meara H,Jorgensen A L,Campbell J,Hobbs M,McCann G,van Staa T,Pirmohamed M]Clin Pharmacol Ther.2013 Dec;94(6):695-701. doi: 10.1038/clpt.2013.161. Epub 2013 Aug 13. PMID: 23942138
67: Should anyone still be taking simvastatin 80 mg?
[Tayal Upasana,Carroll Richard]BMJ Case Rep.2013 Aug 8;2013. pii: bcr2013200415. doi: 10.1136/bcr-2013-200415. PMID: 23929614
68: Rhabdomyolysis and acute kidney injury secondary to interaction between simvastatin and cyclosporine.
[Scarfia Rosalia Viviana,Clementi Anna,Granata Antonio]Ren Fail.2013 Aug;35(7):1056-7. doi: 10.3109/0886022X.2013.810540. PMID: 23859539
69: Statins as a potential risk factor for autoimmune diseases: a case report and review.
[John Santhosh G,Thorn Jennifer,Sobonya Richard]Am J Ther.2014 Jul-Aug;21(4):e94-6. doi: 10.1097/MJT.0b013e31828e5bfb. PMID: 23782756
70: Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study.
[Patel Amit M,Shariff Salimah,Bailey David G,Juurlink David N,Gandhi Sonja,Mamdani Muhammad,Gomes Tara,Fleet Jamie,Hwang Y Joseph,Garg Amit X]Ann Intern Med.2013 Jun 18;158(12):869-76. doi: 10.7326/0003-4819-158-12-201306180-00004. PMID: 23778904
71: Severe rhabdomyolysis associated with concurrent use of simvastatin and sirolimus after cisplatin-based chemotherapy in a kidney transplant recipient.
[Hong Yu Ah,Kim Hyung Duk,Jo Kwanhoon,Park Yun Kyung,Lee Jonghoon,Sun In O,Chung Byung Ha,Park Cheol Whee,Yang Chul Woo,Choi Bum Soon]Exp Clin Transplant.2014 Apr;12(2):152-5. doi: 10.6002/ect.2013.0003. Epub 2013 May 29. PMID: 23734754
72: Protection of rat skeletal muscle fibers by either L-carnitine or coenzyme Q10 against statins toxicity mediated by mitochondrial reactive oxygen generation.
[La Guardia P G,Alberici L C,Ravagnani F G,Catharino R R,Vercesi A E]Front Physiol.2013 May 15;4:103. doi: 10.3389/fphys.2013.00103. eCollection 2013. PMID: 23720630
73: Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors.
[Chauvin Benoit,Drouot Sylvain,Barrail-Tran Aurélie,Taburet Anne-Marie]Clin Pharmacokinet.2013 Oct;52(10):815-31. doi: 10.1007/s40262-013-0075-4. PMID: 23703578
74: Ubiquinol rescues simvastatin-suppression of mitochondrial content, function and metabolism: implications for statin-induced rhabdomyolysis.
[Vaughan Roger A,Garcia-Smith Randi,Bisoffi Marco,Conn Carole A,Trujillo Kristina A]Eur J Pharmacol.2013 Jul 5;711(1-3):1-9. doi: 10.1016/j.ejphar.2013.04.009. Epub 2013 Apr 24. PMID: 23624330
75: Characterization of Statin-Associated Myopathy Case Reports in Thailand Using the Health Product Vigilance Center Database.
[Boonmuang Pornwalai,Nathisuwan Surakit,Chaiyakunapruk Nathorn,Suwankesawong Wimon,Pokhagul Pattreya,Teerawattanapong Nattawat,Supsongserm Pairin]Drug Saf.2013 Sep;36(9):779-87. doi: 10.1007/s40264-013-0055-5. PMID: 23615756
76: Life-threatening rhabdomyolysis following the interaction of two commonly prescribed medications.
[Fallah Alireza,Deep Maitri,Smallwood David,Hughes Peter]Australas Med J.2013 Mar 31;6(3):112-4. doi: 10.4066/AMJ.2013.1616. Print 2013. PMID: 23589735
77: Assessment of statin-associated muscle toxicity in Japan: a cohort study conducted using claims database and laboratory information.
[Chang Chia-Hsien,Kusama Makiko,Ono Shunsuke,Sugiyama Yuichi,Orii Takao,Akazawa Manabu]BMJ Open.2013 Apr 11;3(4). pii: e002040. doi: 10.1136/bmjopen-2012-002040. Print 2013. PMID: 23585384
78: Preparation of calcium phosphate nanocapsules including simvastatin/deoxycholic acid assembly, and their therapeutic effect in osteoporosis model mice.
[Ito Tomoko,Takemasa Manami,Makino Kimiko,Otsuka Makoto]J Pharm Pharmacol.2013 Apr;65(4):494-502. doi: 10.1111/jphp.12008. Epub 2012 Nov 22. PMID: 23488777
79: HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment.
[HPS2-THRIVE Collaborative Group]Eur Heart J.2013 May;34(17):1279-91. doi: 10.1093/eurheartj/eht055. Epub 2013 Feb 26. PMID: 23444397
80: Statins and daptomycin: safety assessment of concurrent use and evaluation of drug interaction liability.
[Golightly Larry K,Barber Gerard R,Barron Michelle A,Page Robert L]Drug Metabol Drug Interact.2013;28(1):49-58. doi: 10.1515/dmdi-2012-0033. PMID: 23314530
81: Rhabdomyolysis caused by an unusual interaction between azithromycin and simvastatin.
[Alreja Gaurav,Inayatullah Saqib,Goel Saurabh,Braden Gregory]J Cardiovasc Dis Res.2012 Oct;3(4):319-22. doi: 10.4103/0975-3583.102720. PMID: 23233778
82: Simvastatin-loaded β-TCP drug delivery system induces bone formation and prevents rhabdomyolysis in OVX mice.
[Chou Joshua,Ito Tomoko,Otsuka Makoto,Ben-Nissan Besim,Milthorpe Bruce]Adv Healthc Mater.2013 May;2(5):678-81. doi: 10.1002/adhm.201200342. Epub 2012 Nov 26. PMID: 23184712
83: Pregabalin and simvastatin: first report of a case of rhabdomyolysis.
[Kaufman Michele B,Choy Mary]P T.2012 Oct;37(10):579-95. PMID: 23115467
84: [Rhabdomyolysis following the coprescription of atorvastatin and fusidic acid].
[Gabignon C,Zeller V,Le Guyader N,Desplaces N,Lidove O,Ziza J-M]Rev Med Interne.2013 Jan;34(1):39-41. doi: 10.1016/j.revmed.2012.09.006. Epub 2012 Oct 24. PMID: 23102978
85: Patient with hypertriglyceridemia, type 2 diabetes, and chronic kidney disease treated with atorvastatin and omega-3 Fatty Acid ethyl esters.
[Athyros Vasilios G,Mikhailidis Dimitri P]Open Cardiovasc Med J.2012;6:122-5. doi: 10.2174/1874192401206010122. Epub 2012 Sep 20. PMID: 23066433
86: Case 7-2012: a man with pain and weakness in the legs.
[Lewis David A]N Engl J Med.2012 Jun 14;366(24):2325-6; author reply 2326. doi: 10.1056/NEJMc1203982#SA1. PMID: 22694015
87: Pravastatin and fenofibrate in combination (Pravafenix(®)) for the treatment of high-risk patients with mixed hyperlipidemia.
[Farnier Michel]Expert Rev Cardiovasc Ther.2012 May;10(5):565-75. doi: 10.1586/erc.12.37. PMID: 22651832
88: Use of administrative data to estimate the incidence of statin-related rhabdomyolysis.
[Floyd James S,Heckbert Susan R,Weiss Noel S,Carrell David S,Psaty Bruce M]JAMA.2012 Apr 18;307(15):1580-2. doi: 10.1001/jama.2012.489. PMID: 22511681
89: Acute rhabdomyolysis caused by combination therapy with atorvastatin and warfarin.
[Mackay J W,Fenech M E,Myint K S]Br J Hosp Med (Lond).2012 Feb;73(2):106-7. PMID: 22504754
90: Statin therapy, myopathy and exercise--a case report.
[Semple Stuart J]Lipids Health Dis.2012 Mar 16;11:40. doi: 10.1186/1476-511X-11-40. PMID: 22420409
91: Case records of the Massachusetts General Hospital. Case 7-2012. A 79-year-old man with pain and weakness in the legs.
[David William S,Chad David A,Kambadakone Avinash,Hedley-Whyte E Tessa]N Engl J Med.2012 Mar 8;366(10):944-54. doi: 10.1056/NEJMcpc1110052. PMID: 22397657
92: Evidence-based prediction of statin use with lipid-panel data from the National Health and Nutrition Examination Survey.
[Gorevski Elizabeth,Bian Boyang,Kelton Christina M L,Martin Boone Jill E,Guo Jeff J]Value Health.2012 Jan;15(1):32-8. doi: 10.1016/j.jval.2011.07.005. Epub 2011 Sep 15. PMID: 22264969
93: Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study.
[Meek Claire,Wierzbicki Anthony S,Jewkes Christina,Twomey Patrick J,Crook Martin A,Jones Alan,Viljoen Adie]Curr Med Res Opin.2012 Mar;28(3):371-8. doi: 10.1185/03007995.2012.657302. Epub 2012 Feb 7. PMID: 22256801
94: Is it OK to eat or drink grapefruit products when I'm taking a statin?
Johns Hopkins Med Lett Health After 50.2011 Dec;23(10):7. PMID: 22216470
95: Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system.
[Sakaeda Toshiyuki,Kadoyama Kaori,Okuno Yasushi]PLoS One.2011;6(12):e28124. doi: 10.1371/journal.pone.0028124. Epub 2011 Dec 20. PMID: 22205938
96: Fatal rhabdomyolysis in a patient with liver cirrhosis after switching from simvastatin to fluvastatin.
[Baek Seung Don,Jang Sun-Joo,Park So-Eun,Ok Tae Jin,Leem Jaechan,Lee Ho-Su,Park So Jung,Kim Tae Hee]J Korean Med Sci.2011 Dec;26(12):1634-7. doi: 10.3346/jkms.2011.26.12.1634. Epub 2011 Nov 29. PMID: 22148003
97: Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes.
[Kwak Hyo-Bum,Thalacker-Mercer Anna,Anderson Ethan J,Lin Chien-Te,Kane Daniel A,Lee Nam-Sihk,Cortright Ronald N,Bamman Marcas M,Neufer P Darrell]Free Radic Biol Med.2012 Jan 1;52(1):198-207. doi: 10.1016/j.freeradbiomed.2011.10.449. Epub 2011 Oct 25. PMID: 22080086
98: [Rhabdomyolysis related to statin and seizures: report of 3 cases].
[Guan Yu-qing,Shi Yan-jie,Wang Qun]Nan Fang Yi Ke Da Xue Xue Bao.2011 Oct;31(10):1795-6. PMID: 22027795
99: Successful re-challenge of daptomycin therapy after initial rhabdomyolysis with co-administration of simvastatin.
[Bland Christopher M,Bookstaver P Brandon,Thomas Sanil]Int J Antimicrob Agents.2011 Dec;38(6):549-50. doi: 10.1016/j.ijantimicag.2011.08.003. Epub 2011 Sep 29. PMID: 21958456
100: Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis.
[Alberton M,Wu P,Druyts E,Briel M,Mills E J]QJM.2012 Feb;105(2):145-57. doi: 10.1093/qjmed/hcr158. Epub 2011 Sep 14. PMID: 21920996
101: Retrospective study on antihyperlipidemic efficacy and safety of simvastatin, ezetimibe and their combination in Korean adults.
[Lee Young-Hee,Kim Mi-Jeong,Choi Chang-Ik,Bae Jung-Woo,Jang Choon-Gon,Lee Seok-Yong]Arch Pharm Res.2011 Aug;34(8):1331-7. doi: 10.1007/s12272-011-0813-9. Epub 2011 Sep 11. PMID: 21910055
102: Evaluation of a pharmacist-managed amiodarone monitoring program.
[Spence Michele M,Polzin Jennifer K,Weisberger Calvin L,Martin John P,Rho Jay P,Willick Giselle H]J Manag Care Pharm.2011 Sep;17(7):513-22. PMID: 21870892
103: Statins for acute ischemic stroke.
[Squizzato Alessandro,Romualdi Erica,Dentali Francesco,Ageno Walter]Cochrane Database Syst Rev.2011 Aug 10;(8):CD007551. doi: 10.1002/14651858.CD007551.pub2. PMID: 21833959
104: Effects of myosin heavy chain (MHC) plasticity induced by HMGCoA-reductase inhibition on skeletal muscle functions.
[Trapani Laura,Melli Luca,Segatto Marco,Trezza Viviana,Campolongo Patrizia,Jozwiak Adam,Swiezewska Ewa,Pucillo Leopoldo Paolo,Moreno Sandra,Fanelli Francesca,Linari Marco,Pallottini Valentina]FASEB J.2011 Nov;25(11):4037-47. doi: 10.1096/fj.11-184218. Epub 2011 Jul 28. PMID: 21798954
105: Simvastatin as add-on therapy to interferon β-1a for relapsing-remitting multiple sclerosis (SIMCOMBIN study): a placebo-controlled randomised phase 4 trial.
[Sorensen Per Soelberg,Lycke Jan,Erälinna Juha-Pekka,Edland Astrid,Wu Xingchen,Frederiksen Jette Lautrup,Oturai Annette,Malmeström Clas,Stenager Egon,Sellebjerg Finn,Sondergaard Helle Bach,SIMCOMBIN study investigators]Lancet Neurol.2011 Aug;10(8):691-701. doi: 10.1016/S1474-4422(11)70144-2. PMID: 21742556
106: Reversal of drug-induced rhabdomyolysis on bone scan.
[Abrams Joseph,Tiu Serafin]Clin Nucl Med.2011 Aug;36(8):e101-2. doi: 10.1097/RLU.0b013e31821c9ac1. PMID: 21716004
107: Concomitant use of simvastatin and amiodarone resulting in severe rhabdomyolysis: a case report and review of the literature.
[Marot A,Morelle J,Chouinard V A,Jadoul M,Lambert M,Demoulin N]Acta Clin Belg.2011 Mar-Apr;66(2):134-6. PMID: 21630612
108: Rhabdomyolysis-induced acute renal failure due to itraconazole and simvastatin association.
[Roques Sébastien,Lytrivi Maria,Rusu Daniel,Devriendt Jacques,De Bels David]Drug Metabol Drug Interact.2011;26(2):79-80. doi: 10.1515/DMDI.2011.106. Epub 2011 Apr 18. PMID: 21495875
109: Are all statins the same? Focus on the efficacy and tolerability of pitavastatin.
[da Silva Pedro Marques]Am J Cardiovasc Drugs.2011;11(2):93-107. doi: 10.2165/11591190-000000000-00000. PMID: 21446776
110: Rhabdomyolysis in a Prostate Cancer Patient Taking Ketoconazole and Simvastatin: Case Report and Review of the Literature.
[Watkins Jack L,Atkinson Bradley J,Pagliaro Lance C]Ann Pharmacother.2011 Feb;45(2):e9. doi: 10.1345/aph.1P433. PMID: 21304039
111: [Genetic marker of statin-induced rhabdomyolysis].
[Chiba Kan,Morimoto Kaori]Yakugaku Zasshi.2011 Feb;131(2):247-53. PMID: 21297370
112: By the way, doctor. I've been advised not to take my statin drug, simvastatin, with grapefruit juice. But is it safe to take the medication at night and then drink grapefruit juice in the morning?
[Robb-Nicholson Celeste]Harv Womens Health Watch.2010 Oct;18(2):8. PMID: 21268318
113: [High dose of simvastatin to minimize the risk of myopathy?].
[Frey Otto]Med Monatsschr Pharm.2010 Sep;33(9):354-5. PMID: 21192445
114: Rhabdomyolysis with simvastatin.
[Patel Brijesh R,Choudhury Maitrayee]BMJ Case Rep.2011 Jan 11;2011. pii: bcr1220092552. doi: 10.1136/bcr.12.2009.2552. PMID: 22715232
115: Statin-associated rhabdomyolysis with acute renal failure complicated by intradialytic NSTEMI: a review of lipid management considerations.
[Kar Subrata,Chockalingam Anand]Am J Ther.2013 Jan;20(1):57-60. doi: 10.1097/MJT.0b013e3181ff7c79. PMID: 21192242
116: [Complete atrioventricular block due to hyperkalemia caused by rhabdomyolysis during treatment with statin].
[Grabka Marek,Wita Krystian,Berger-Kucza Adrianna,Bochenek Tomasz,Turski Maciej,Trusz-Gluza Maria]Kardiol Pol.2010 Dec;68(12):1376-8; discussion 1379. PMID: 21174295
117: [88 years old woman with acute muscular weakness and diffuse muscular pain: have you thought about the drugs?].
[Arnold Ch,Lamy O,Hagmann N]Praxis (Bern 1994).2010 Dec 1;99(24):1507-11. doi: 10.1024/1661-8157/a000324. PMID: 21125536
118: Rhabdomyolysis following co-prescription of fusidic acid and atorvastatin.
[Teckchandani S,Robertson S,Almond A,Donaldson K,Isles C]J R Coll Physicians Edinb.2010 Mar;40(1):33-6. doi: 10.4997/JRCPE.2010.108. PMID: 21125037
119: Rhabdomyolysis in a patient receiving high-dose simvastatin after the induction of therapeutic hypothermia.
[Dearing Natalie,Norgard Nicholas B]Ann Pharmacother.2010 Dec;44(12):1994-7. doi: 10.1345/aph.1P352. Epub 2010 Nov 2. PMID: 21045169
120: Rhabdomyolysis in a patient receiving ranolazine and simvastatin.
[Hylton Ann C,Ezekiel Tanya O]Am J Health Syst Pharm.2010 Nov 1;67(21):1829-31. doi: 10.2146/ajhp090299. PMID: 20966146
121: Statins alter intracellular calcium homeostasis in malignant hyperthermia susceptible individuals.
[Metterlein T,Schuster F,Tadda L,Hager M,Roewer N,Anetseder M]Cardiovasc Ther.2010 Dec;28(6):356-60. doi: 10.1111/j.1755-5922.2010.00237.x. Epub 2010 Oct 19. PMID: 20955541
122: Results of a safety initiative for patients on concomitant amiodarone and simvastatin therapy in a Veterans Affairs medical center.
[Karimi Sahar,Hough Augustus,Beckey Cherylyn,Parra David]J Manag Care Pharm.2010 Sep;16(7):472-81. PMID: 20726676
123: Concomitant administration of simvastatin and danazol associated with fatal rhabdomyolysis.
[Stankovic Ivan,Vlahovic-Stipac Alja,Putnikovic Biljana,Cvetkovic Zorica,Neskovic Aleksandar N]Clin Ther.2010 May;32(5):909-14. doi: 10.1016/j.clinthera.2010.04.017. PMID: 20685498
124: The risk for significant creatine kinase elevation with statins.
[Stolcpart Ryan S,Olson Kari L,Delate Thomas,Rasmussen Jon,Rehring Thomas F,Merenich John A]Am J Cardiovasc Drugs.2010;10(3):187-92. doi: 10.2165/11536130-000000000-00000. PMID: 20524720
125: Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double-blind study.
[Roth Eli M,McKenney James M,Kelly Maureen T,Setze Carolyn M,Carlson Dawn M,Gold Alex,Stolzenbach James C,Williams Laura A,Jones Peter H]Am J Cardiovasc Drugs.2010;10(3):175-86. doi: 10.2165/11533430-000000000-00000. PMID: 20524719
126: Simvastatin-induced myoglobinuric acute kidney injury following ciclosporin treatment for alopecia universalis.
[Teutonico Annalisa,Libutti Pasquale,Lomonte Carlo,Basile Carlo]NDT Plus.2010 Jun;3(3):273-275. doi: 10.1093/ndtplus/sfq012. Epub 2010 Feb 28. PMID: 28657046
127: [Drug interaction caused by communication problems. Rhabdomyolysis due to a combination of itraconazole and simvastatin].
[Tiessen Renger G,Lagerwey Hendrik Jan G,Jager Gea J,Sprenger Herman G]Ned Tijdschr Geneeskd.2010;154:A762. PMID: 20456775
128: Major diet-drug interactions affecting the kinetic characteristics and hypolipidaemic properties of statins.
[Vaquero M P,Sánchez Muniz F J,Jiménez Redondo S,Prats Oliván P,Higueras F J,Bastida S]Nutr Hosp.2010 Mar-Apr;25(2):193-206. PMID: 20449528
129: [Renal failure due to simvastatin-induced rhabdomyolysis in a patient with subclinical hypothyroidism].
[Requena Carrión E,Ayala Jiménez L,Sierra García F]Farm Hosp.2010 Jan-Feb;34(1):45-6. doi: 10.1016/j.farma.2009.08.001. Epub 2010 Jan 19. PMID: 20144822
130: Long-term treatment with pitavastatin is effective and well tolerated by patients with primary hypercholesterolemia or combined dyslipidemia.
[Ose Leiv,Budinski Dragos,Hounslow Neil,Arneson Valerie]Atherosclerosis.2010 May;210(1):202-8. doi: 10.1016/j.atherosclerosis.2009.12.009. Epub 2009 Dec 11. PMID: 20080236
131: Rhabdomyolysis with acute renal failure triggered by the seasonal flu vaccination in a patient taking simvastatin.
[Shah S V,Reddy K]BMJ Case Rep.2010 Oct 4;2010. pii: bcr1120092485. doi: 10.1136/bcr.11.2009.2485. PMID: 22778082
132: Year two assessment of fenofibric acid and moderate-dose statin combination: a phase 3, open-label, extension study.
[Kipnes Mark S,Roth Eli M,Rhyne James M,Setze Carolyn M,Lele Aditya,Kelly Maureen T,Sleep Darryl J,Stolzenbach James C]Clin Drug Investig.2010;30(1):51-61. doi: 10.2165/11319800-000000000-00000. PMID: 19995098
133: Neuroleptic malignant syndrome or a statin drug reaction? A case report.
[Cooper Joyce M,Jones Alison L]Clin Neuropharmacol.2009 Nov-Dec;32(6):348-9. doi: 10.1097/WNF.0b013e3181acc92d. PMID: 19952877
134: Does simvastatin cause more myotoxicity compared with other statins?
[Backes James M,Howard Patricia A,Ruisinger Janelle F,Moriarty Patrick M]Ann Pharmacother.2009 Dec;43(12):2012-20. doi: 10.1345/aph.1M410. Epub 2009 Nov 17. PMID: 19920157
135: Plasmapheresis in a patient with rhabdomyolysis: a case report.
[Swaroop Rohina,Zabaneh Raja,Parimoo Nakul]Cases J.2009 Aug 12;2:8138. doi: 10.4076/1757-1626-2-8138. PMID: 19918458
136: [Lower and lower cholesterol targets increase adverse effects].
[Hjemdahl Paul,Allhammar Anette,Heaton Claire,Hulting Johan,Kahan Thomas,Malmström Rickard,Martinsson Arne,Rücker Franz,Schenck-Gustafsson Karin,Schwieler Jonas,Törnerud Mattias,Wettermark Björn,Forfattarna utgör Läkemedelssakkunnigas (Läksak) expert-grupp för hjärt-kärlsjukdomar,,Stockholms Iäns landsting]Lakartidningen.2009 Sep 30-Oct 6;106(40):2550-1. PMID: 19908628
137: [Warning for simvastatin 80 mg!].
[Olsson Anders G]Lakartidningen.2009 Sep 30-Oct 6;106(40):2549-50. PMID: 19908627
138: Decreased ubiquinone availability and impaired mitochondrial cytochrome oxidase activity associated with statin treatment.
[Duncan Andrew J,Hargreaves Iain P,Damian Maxwell S,Land John M,Heales Simon J R]Toxicol Mech Methods.2009 Jan;19(1):44-50. doi: 10.1080/15376510802305047. PMID: 19778232
139: Rhabdomyolysis a result of azithromycin and statins: an unrecognized interaction.
[Strandell Johanna,Bate Andrew,Hägg Staffan,Edwards I Ralph]Br J Clin Pharmacol.2009 Sep;68(3):427-34. doi: 10.1111/j.1365-2125.2009.03473.x. PMID: 19740401
140: Short term treatment with clarithromycin resulting in colchicine-induced rhabdomyolysis.
[McKinnell James,Tayek John A]J Clin Rheumatol.2009 Sep;15(6):303-5. doi: 10.1097/RHU.0b013e3181bbbcd7. PMID: 19734738
141: Recovery time in a case of gemfibrozil and simvastatin-associated rhabdomyolysis.
[Cummins Dosha,Mackey Michael,Baker Erica]South Med J.2009 Aug;102(8):858-60. doi: 10.1097/SMJ.0b013e3181ad6078. PMID: 19593291
142: Concurrent use of statins and amiodarone.
[Borders-Hemphill Vicky]Consult Pharm.2009 May;24(5):372-9. PMID: 19555146
143: [Adverse muscle effects of a podofyllotoxin-containing cytotoxic drug product with simvastatin].
[Kaipiainen-Seppänen Oili,Savolainen Elina,Elfving Pia,Kononoff Aulikki]Duodecim.2009;125(7):788-91. PMID: 19432085
144: Severe rhabdomyolysis and acute renal failure secondary to concomitant use of simvastatin with rapamycin plus tacrolimus in liver transplant patient.
[Dopazo C,Bilbao I,Lázaro J L,Sapisochin G,Caralt M,Blanco L,Castells L,Charco R]Transplant Proc.2009 Apr;41(3):1021-4. doi: 10.1016/j.transproceed.2009.02.019. PMID: 19376416
145: Efficacy and safety of fenofibric acid in combination with a statin in patients with mixed dyslipidemia: Pooled analysis of three phase 3, 12-week randomized, controlled studies.
[Jones Peter H,Davidson Michael H,Goldberg Anne C,Pepine Carl J,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Lele Aditya,Sleep Darryl J,Stolzenbach James C]J Clin Lipidol.2009 Apr;3(2):125-37. doi: 10.1016/j.jacl.2009.02.007. Epub 2009 Feb 11. PMID: 21291802
146: Rhabdomyolysis caused by co-medication with simvastatin and clarithromycin.
[Wagner Judith,Suessmair Christine,Pfister Hans-Walter]J Neurol.2009 Jul;256(7):1182-3. doi: 10.1007/s00415-009-5078-6. Epub 2009 Mar 1. PMID: 19252767
147: Renal failure and rhabdomyolysis associated with sitagliptin and simvastatin use. But what about the amiodarone?
[Boucher B J]Diabet Med.2009 Feb;26(2):192-3. doi: 10.1111/j.1464-5491.2008.02659.x. PMID: 19236630
148: Rhabdomyolysis reports show interaction between simvastatin and CYP3A4 inhibitors.
[Rowan Christopher,Brinker Allen D,Nourjah Parivash,Chang Jennie,Mosholder Andrew,Barrett Jeffrey S,Avigan Mark]Pharmacoepidemiol Drug Saf.2009 Apr;18(4):301-9. doi: 10.1002/pds.1711. PMID: 19206087
149: [Maximal initial dose of simvastatin causing acute renal failure through rhabdomyolysis: risk factors, pathomechanism and therapy related to a case].
[Deme Dániel,Al-Hadad Aref,Varga Tünde,Szántó Erika,Sándor Katalin,Rakonczai Ervin]Orv Hetil.2009 Feb 8;150(6):265-9. doi: 10.1556/OH.2009.28498. PMID: 19179259
150: [Statin therapy and muscle disorders].
[Abel Tatjána,Fehér János]Orv Hetil.2009 Feb 8;150(6):261-3. doi: 10.1556/OH.2009.28520. PMID: 19179258
151: Researchers worry about myopathy risk for patients taking high-dose simvastatin.
[Mitka Mike]JAMA.2009 Jan 21;301(3):261-2. doi: 10.1001/jama.2008.939. PMID: 19155447
152: Should high creatine kinase discourage the initiation or continuance of statins for the treatment of hypercholesterolemia?
[Glueck Charles J,Rawal Bishal,Khan Naseer Ahmed,Yeramaneni Samrat,Goldenberg Naila,Wang Ping]Metabolism.2009 Feb;58(2):233-8. doi: 10.1016/j.metabol.2008.09.019. PMID: 19154957
153: Rhabdomyolysis due to an uncommon interaction of ciprofloxacin with simvastatin.
[Sawant R D]Can J Clin Pharmacol.2009 Winter;16(1):e78-9. Epub 2009 Jan 16. PMID: 19151423
154: Possible mechanisms underlying statin-induced skeletal muscle toxicity in L6 fibroblasts and in rats.
[Itagaki Mai,Takaguri Akira,Kano Seiichiro,Kaneta Shigeru,Ichihara Kazuo,Satoh Kumi]J Pharmacol Sci.2009 Jan;109(1):94-101. Epub 2009 Jan 8. PMID: 19129682
155: Rhabdomyolysis caused by an interaction of simvastatin and fusidic acid.
[Herring Roselle,Caldwell Gordon,Wade Stuart]BMJ Case Rep.2009;2009. pii: bcr03.2009.1722. doi: 10.1136/bcr.03.2009.1722. Epub 2009 Sep 20. PMID: 21931583
156: Severe rhabdomyolysis and acute renal failure secondary to use of simvastatin in undiagnosed hypothyroidism.
[Qari Faiza A]Saudi J Kidney Dis Transpl.2009 Jan;20(1):127-9. PMID: 19112232
157: Myotonic potentials in statin-induced rhabdomyolysis.
[de Almeida Diogo Fraxino,Lissa Terezinha Valente,Melo Aluísio Cláudio Mentor Neves Couto]Arq Neuropsiquiatr.2008 Dec;66(4):891-3. PMID: 19099134
158: Multiple drug interactions in a renal transplant patient leading to simvastatin-induced rhabdomyolysis: a case report.
[Rifkin Stephen I]Medscape J Med.2008;10(11):264. Epub 2008 Nov 19. PMID: 19099014
159: [Rhabdomyolysis and renal failure secondary to interaction between simvastatin, ciclosporin A and risperidone in an allogeneic stem cell transplantation patient].
[Vives Susana,Batlle Montserrat,Montané Eva,Ribera Josep-María]Med Clin (Barc).2008 Nov 15;131(17):676. PMID: 19087798
160: A case of asymptomatic cytoplasmic body myopathy revealed by sinvastatin.
[Evangelista Teresinha,Ferro José,Pereira Pedro,de Carvalho Mamede]Neuromuscul Disord.2009 Jan;19(1):66-8. doi: 10.1016/j.nmd.2008.10.008. Epub 2008 Dec 11. PMID: 19084404
161: Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: a phase 3, randomized, controlled study.
[Mohiuddin Syed M,Pepine Carl J,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Sleep Darryl J,Stolzenbach James C]Am Heart J.2009 Jan;157(1):195-203. doi: 10.1016/j.ahj.2008.08.027. Epub 2008 Nov 20. PMID: 19081418
162: Renal failure and rhabdomyolysis associated with sitagliptin and simvastatin use.
[Kao D P,Kohrt H E,Kugler J]Diabet Med.2008 Oct;25(10):1229-30. doi: 10.1111/j.1464-5491.2008.02536.x. PMID: 19046202
163: Long-term safety and efficacy of fenofibric acid in combination with statin therapy for the treatment of patients with mixed dyslipidemia.
[Bays Harold E,Jones Peter H,Mohiuddin Syed M,Kelly Maureen T,Sun Hsiaoming,Setze Carolyn M,Buttler Susan M,Sleep Darryl J,Stolzenbach James C]J Clin Lipidol.2008 Dec;2(6):426-35. doi: 10.1016/j.jacl.2008.10.001. Epub 2008 Nov 12. PMID: 21291776
164: Interpretation of creatine kinase and aldolase for statin-induced myopathy: Reliance on serial testing based on biological variation.
[Wu Alan H B,Smith Andrew,Wians Frank]Clin Chim Acta.2009 Jan;399(1-2):109-11. doi: 10.1016/j.cca.2008.09.023. Epub 2008 Sep 26. PMID: 18848535
165: Clinical reasoning: rhabdomyolysis after combined treatment with simvastatin and fluconazole.
[Findling O,Meier N,Sellner J,Nedeltchev K,Arnold M]Neurology.2008 Oct 7;71(15):e34-7. doi: 10.1212/01.wnl.0000327566.57661.09. PMID: 18838657
166: Relative safety profiles of high dose statin regimens.
[Escobar Carlos,Echarri Rocio,Barrios Vivencio]Vasc Health Risk Manag.2008;4(3):525-33. PMID: 18827903
167: [Bilateral leg compartment syndrome due to severe myonecrosis caused by inappropriate use of simvastatin].
[Chochola M,Lubanda J-C,Skalicka L,Varejka P,Horejs J,Prskavec T,Balík M,Semrád M,Linhart A]J Mal Vasc.2008 Dec;33(4-5):229-33. doi: 10.1016/j.jmv.2008.07.092. Epub 2008 Sep 25. PMID: 18819764
168: These drugs don't mix.
Nursing.2008 Oct;38(10):12. doi: 10.1097/01.NURSE.0000337211.71313.95. PMID: 18812982
169: [Case of severe rhabdomyolosis after protracted overdose of simvastatin].
[Greve Tine]Ugeskr Laeger.2008 Sep 15;170(38):2981. PMID: 18808756
170: Long-term (48-week) safety of ezetimibe 10 mg/day coadministered with simvastatin compared to simvastatin alone in patients with primary hypercholesterolemia.
[Bays Harold,Sapre Aditi,Taggart William,Liu Ji,Capece Rachel,Tershakovec Andrew]Curr Med Res Opin.2008 Oct;24(10):2953-66. doi: 10.1185/03007990802365094 . Epub 2008 Sep 8. PMID: 18782465
171: Combination drug products: an indication for medication reconciliation and pharmacist counseling.
[Stroup Jeffrey,Stephens Johnny]J Am Pharm Assoc (2003).2008 Jul-Aug;48(4):541-3. doi: 10.1331/JAPhA.2008.07058. PMID: 18653432
172: Pharmacogenomics and drug toxicity.
[Nakamura Yusuke]N Engl J Med.2008 Aug 21;359(8):856-8. doi: 10.1056/NEJMe0805136. Epub 2008 Jul 23. PMID: 18650508
173: Rhabdomyolysis resulting from pharmacologic interaction between erlotinib and simvastatin.
[Veeraputhiran Muthu,Sundermeyer Mark]Clin Lung Cancer.2008 Jul;9(4):232-4. doi: 10.3816/CLC.2008.n.036. PMID: 18650173
174: Simvastatin-induced rhabdomyolysis and acute renal injury.
[Waness Abdelkarim,Bahlas Sami,Al Shohaib Saad]Blood Purif.2008;26(4):394-8. doi: 10.1159/000141931. Epub 2008 Jul 1. PMID: 18594138
175: Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors.
[Molden Espen,Skovlund Eva,Braathen Pia]Drug Saf.2008;31(7):587-96. PMID: 18558792
176: [Creatine kinase increase under simvastatin--therapeutic consequences?].
[Rasche-Schürmann Celine C,Picksak Gesine,Stichtenoth Dirk O]Med Monatsschr Pharm.2008 Jan;31(1):25-7. PMID: 18522017
177: Rhabdomyolysis after ezetimibe/simvastatin therapy in an HIV-infected patient.
[Chanson Noemie,Bossi Philippe,Schneider Luminita,Bourry Edward,Izzedine Hassane]NDT Plus.2008 Jun;1(3):157-61. doi: 10.1093/ndtplus/sfn012. PMID: 25983864
178: A case of recurrent rapidly progressive lower limb weakness.
[Mahmoud Kamala,D'Costa Domnick,Dorrian Sue]Age Ageing.2008 Jul;37(4):483. doi: 10.1093/ageing/afn104. Epub 2008 May 16. PMID: 18487263
179: Rhabdomyolysis induced by simvastatin-fluconazole combination.
[Hazin Ribhi,Abuzetun Jamil Y,Suker Manar,Porter Joann]J Natl Med Assoc.2008 Apr;100(4):444-6. PMID: 18481486
180: Presumed interaction of fusidic acid with simvastatin.
[Burtenshaw A J,Sellors G,Downing R]Anaesthesia.2008 Jun;63(6):656-8. doi: 10.1111/j.1365-2044.2007.05434.x. PMID: 18477279
181: Tendinous disorders attributed to statins: a study on ninety-six spontaneous reports in the period 1990-2005 and review of the literature.
[Marie Isabelle,Delafenêtre Hélène,Massy Nathalie,Thuillez Christian,Noblet Catherine,Network of the French Pharmacovigilance Centers]Arthritis Rheum.2008 Mar 15;59(3):367-72. doi: 10.1002/art.23309. PMID: 18311771
182: Incidence of adverse events with HMG-CoA reductase inhibitors in liver transplant patients.
[Martin Jill E,Cavanaugh Teresa M,Trumbull Leslie,Bass Maryetta,Weber Fredrick,Aranda-Michel Jaime,Hanaway Michael,Rudich Steven]Clin Transplant.2008 Jan-Feb;22(1):113-9. doi: 10.1111/j.1399-0012.2007.00780.x. PMID: 18217912
183: Estimating the extent of reporting to FDA: a case study of statin-associated rhabdomyolysis.
[McAdams Mara,Staffa Judy,Dal Pan Gerald]Pharmacoepidemiol Drug Saf.2008 Mar;17(3):229-39. doi: 10.1002/pds.1535. PMID: 18175291
184: Severe rhabdomyolysis and acute renal failure secondary to the use of simvastatin in undiagnosed hypothyroidism.
[Qari F A]Indian J Nephrol.2008 Jan;18(1):28-9. doi: 10.4103/0971-4065.41287. PMID: 20368919
185: On call. Statins and muscle damage.
[Simon Harvey B]Harv Mens Health Watch.2007 Aug;12(1):8. PMID: 18018322
186: Acute renal failure and myalgia in a transplant patient.
[Najafian Behzad,Franklin Donald B,Fogo Agnes B]J Am Soc Nephrol.2007 Nov;18(11):2870-4. Epub 2007 Oct 17. PMID: 17942960
187: [Rhabdomyolysis caused by the association of simvastatin and risperidone].
[Patier José Luis,Ferrere Federico,Moreno-Cobo María Angeles,Echaniz Ana]Med Clin (Barc).2007 Sep 29;129(11):439. PMID: 17927942
188: Rapid onset of muscle weakness (rhabdomyolysis) associated with the combined use of simvastatin and colchicine.
[Justiniano Maria,Dold Sylvia,Espinoza Luis R]J Clin Rheumatol.2007 Oct;13(5):266-8. PMID: 17921794
189: A cautionary tale: delayed onset rhabdomyolysis due to erythromycin/simvastatin interaction.
[Campbell G,Jayakumar U,McCracken S,Bene J]Age Ageing.2007 Sep;36(5):597. PMID: 17913761
190: Simvastatin-induced rhabdomyolysis following cyclosporine treatment for uveitis.
[Lasocki Anita,Vote Brendan,Fassett Robert,Zamir Ehud]Ocul Immunol Inflamm.2007 Jul-Aug;15(4):345-6. PMID: 17763133
191: Rhabdomyolysis after addition of digitoxin to chronic simvastatin and amiodarone therapy.
[Nägele H,Behrens S,Hashagen S,Azizi M]Drug Metabol Drug Interact.2007;22(2-3):195-200. PMID: 17708069
192: CYP2D6*4 polymorphism is associated with statin-induced muscle effects.
[Frudakis Tony N,Thomas Matthew J,Ginjupalli Siva N,Handelin Barbara,Gabriel Richard,Gomez Hector J]Pharmacogenet Genomics.2007 Sep;17(9):695-707. PMID: 17700359
193: Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials.
[Alsheikh-Ali Alawi A,Maddukuri Prasad V,Han Hui,Karas Richard H]J Am Coll Cardiol.2007 Jul 31;50(5):409-18. Epub 2007 Jul 16. PMID: 17662392
194: Fatal toxic myopathy attributed to propofol, methylprednisolone, and cyclosporine after prior exposure to colchicine and simvastatin.
[Francis Lisa,Bonilla Eduardo,Soforo Ekaterina,Neupane Hom,Nakhla Hassan,Fuller Christine,Perl Andras]Clin Rheumatol.2008 Jan;27(1):129-31. Epub 2007 Jul 13. PMID: 17628739
195: Severe rhabdomyolysis and acute renal failure secondary to concomitant use of simvastatin, amiodarone, and atazanavir.
[Schmidt Ginelle A,Hoehns James D,Purcell Jessica L,Friedman Robert L,Elhawi Yasir]J Am Board Fam Med.2007 Jul-Aug;20(4):411-6. PMID: 17615423
196: [Interactions between statins and macrolide antibiotics].
[Molden Espen,Andersson Kirsti Svendsen,Jacobsen Dag]Tidsskr Nor Laegeforen.2007 Jun 14;127(12):1660-1. PMID: 17571108
197: Therapeutic rationale of combining therapy with gemfibrozil and simvastatin.
[Curtin Patrick O,Jones William N]J Am Pharm Assoc (2003).2007 Mar-Apr;47(2):140-6. PMID: 17510000
198: Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy.
[Silva Matthew,Matthews Michele L,Jarvis Courtney,Nolan Nicole M,Belliveau Paul,Malloy Michael,Gandhi Pritesh]Clin Ther.2007 Feb;29(2):253-60. PMID: 17472818
199: Safety of aggressive lipid management.
[Davidson Michael H,Robinson Jennifer G]J Am Coll Cardiol.2007 May 1;49(17):1753-62. Epub 2007 Apr 16. PMID: 17466224
200: Rosuvastatin: renal disorders and rhabdomyolysis.
Prescrire Int.2007 Apr;16(88):68-9. PMID: 17458050
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.