Search for drugs:
Typing the drug name to query
TENOFOVIR DISOPROXIL FUMARATE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of VIREAD. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Immune System Disorders
- allergic reaction, including angioedema
- Metabolism and Nutrition Disorders
- lactic acidosis, hypokalemia, hypophosphatemia
- Respiratory, Thoracic, and Mediastinal Disorders
- dyspnea
- Gastrointestinal Disorders
- pancreatitis, increased amylase, abdominal pain
- Hepatobiliary Disorders
- hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT gamma GT)
- Skin and Subcutaneous Tissue Disorders
- rash
- Musculoskeletal and Connective Tissue Disorders
- rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
- Renal and Urinary Disorders
- acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuria
- General Disorders and Administration Site Conditions
- asthenia
- The following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
142
42770
Other ADRs
18353
14098926
Odds Ratio = 2.551
Drug Property Information
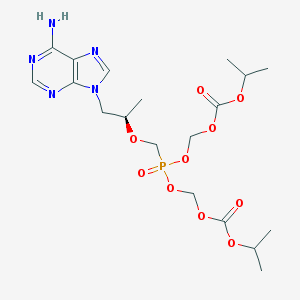
SMILE Code:
C[C@H](CN1C=NC2=C1N=CN=C2N)OCP(=O)(OCOC(=O)OC(C)C)OCOC(=O)OC(C)C
C[C@H](CN1C=NC2=C1N=CN=C2N)OCP(=O)(OCOC(=O)OC(C)C)OCOC(=O)OC(C)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: A 68-year old male presenting with rhabdomyolysis-associated acute kidney injury following concomitant use of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate and pravastatin/fenofibrate: a case report.
[Suttels Veronique,Florence Eric,Leys John,Vekemans Marc,Van den Ende Jef,Vlieghe Erika,Kenyon Chris]J Med Case Rep.2015 Sep 8;9:190. doi: 10.1186/s13256-015-0671-z. PMID: 26347243
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.