Search for drugs:
Typing the drug name to query
METHYLPHENIDATE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:2
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post approval use of methylphenidate products. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions are as follows:
- Blood and Lymphatic System Disorders: Pancytopenia, Thrombocytopenia, Thrombocytopenic purpura
- Cardiac Disorders: Angina pectoris, Bradycardia, Extrasystole, Supraventricular tachycardia, Ventricular extrasystole
- Eye Disorders: Diplopia, Mydriasis, Visual impairment
- General Disorders: Chest pain, Chest discomfort, Hyperpyrexia
- Immune System Disorders: Hypersensitivity reactions such as Angioedema, Anaphylactic reactions, Auricular swelling, Bullous conditions, Exfoliative conditions, Urticarias, Pruritis NEC, Rashes, Eruptions, and Exanthemas NEC
- Investigations: Alkaline phosphatase increased, Bilirubin increased, Hepatic enzyme increased, Platelet count decreased, White blood cell count abnormal
- Musculoskeletal, Connective Tissue and Bone Disorders: Arthralgia, Myalgia, Muscle twitching, Rhabdomyolysis
- Nervous System Disorders: Convulsion, Grand mal convulsion, Dyskinesia, Serotonin syndrome in combination with serotonergic drugs
- Psychiatric Disorders: Disorientation, Hallucination, Hallucination auditory, Hallucination visual, Libido changes, Mania
- Urogenital System: Priapism
- Skin and Subcutaneous Tissue Disorders: Alopecia, Erythema
- Vascular Disorders: Raynaud's phenomenon
- OVERDOSAGE
- Signs and Symptoms
- Signs and symptoms of acute methylphenidate overdosage, resulting principally from overstimulation of the CNS and from excessive sympathomimetic effects, may include the following: nausea, vomiting, diarrhea, restlessness, anxiety, agitation, tremors, hyperflexia, muscle twitching, convulsion (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, hypotension, tachypnea, mydriasis, dryness of mucous membranes, and rhabdomyolysis.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
3
42909
Other ADRs
13738
14103541
Odds Ratio = 0.072
Drug Property Information
ATC Code(s):
- N06BA04 - methylphenidate
- N06BA - Centrally acting sympathomimetics
- N06B - "PSYCHOSTIMULANTS, AGENTS USED FOR ADHD AND NOOTROPICS"
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:methylphenidate
Active Ingredient UNII:207ZZ9QZ49
Drugbank ID:DB00422
PubChem Compound:4158
CAS Number:20748-11-2
Dosage Form(s):tablet, orally disintegrating
Route(s) Of Administrator:oral
Daily Dose:
- 30.0 mg/day N06BA04
Chemical Structure: 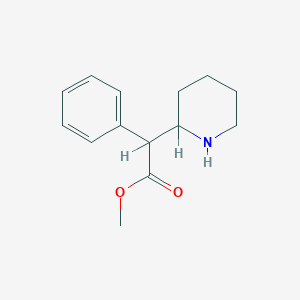

SMILE Code:
COC(=O)C(C1CCCCN1)C2=CC=CC=C2
COC(=O)C(C1CCCCN1)C2=CC=CC=C2
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Trimethoprim-Sulfamethoxazole-Induced Rhabdomyolysis; Gabapentin-Induced Hypoglycemia in Diabetic and Nondiabetic Patients; Purple Glove Syndrome After Oral Phenytoin Administration; Acute Dystonic Reaction After Methylphenidate Initiation; Serotonin Syndrome with Vilazodone Monotherapy; Cabozantinib-Associated Dermatologic Adverse Reactions.
[Mancano Michael A]Hosp Pharm.2015 Sep;50(8):662-6. doi: 10.1310/hpj5008-662. Epub 2015 Sep 16. PMID: 26715798
2: Serotonin syndrome.
[Bodner R A,Lynch T,Lewis L,Kahn D]Neurology.1995 Feb;45(2):219-23. PMID: 7854515
3: Pemoline-induced choreoathetosis and rhabdomyolysis.
[Briscoe J G,Curry S C,Gerkin R D,Ruiz R R]Med Toxicol Adverse Drug Exp.1988 Jan-Dec;3(1):72-6. PMID: 3367787
4: Medical complications of drug abuse.
[Becker C E]Adv Intern Med.1979;24:183-202. PMID: 371359
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.