Search for drugs:
Typing the drug name to query
CLOZAPINE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation
- QT prolongation, Torsade de Pointes and other life-threatening ventricular arrhythmias, cardiac arrest, and sudden death have occurred with clozapine treatment. When prescribing clozapine, consider the presence of additional risk factors for QT prolongation and serious cardiovascular reactions. Conditions that increase these risks include the following: history of QT prolongation, long QT syndrome, family history of long QT syndrome or sudden cardiac death, significant cardiac arrhythmia, recent myocardial infarction, uncompensated heart failure, treatment with other medications that cause QT prolongation, treatment with medications that inhibit the metabolism of clozapine, and electrolyte abnormalities.
- Prior to initiating treatment with clozapine, perform a careful physical examination, medical history, and concomitant medication history. Consider obtaining a baseline ECG and serum chemistry panel. Correct electrolyte abnormalities. Discontinue clozapine if the QTc interval exceeds 500 msec. If patients experience symptoms consistent with Torsades de Pointes, or other arrhythmias (e.g., syncope, presyncope, dizziness, or palpitations), obtain a cardiac evaluation and discontinue clozapine.
- Use caution when administering concomitant medications that prolong the QT interval or inhibit the metabolism of clozapine. Drugs that cause QT prolongation include: specific antipsychotics (e.g., ziprasidone, iloperidone, chlorpromazine, thioridazine, mesoridazine, droperidol, pimozide), specific antibiotics (e.g., erythromycin, gatifloxacin, moxifloxacin, sparfloxacin), Class 1A antiarrhythmic medications (e.g., quinidine, procainamide) or Class III antiarrhythmics (e.g., amiodarone, sotalol), and others (e.g., pentamidine, levomethadyl acetate, methadone, halofantrine, mefloquine, dolasetron mesylate, probucol or tacrolimus). Clozapine is primarily metabolized by CYP isoenzymes 1A2, 2D6, and 3A4. Concomitant treatment with inhibitors of these enzymes can increase the concentration of clozapine [see DRUG INTERACTIONS (7.1) and CLINICAL PHARMACOLOGY (12.3)].
- Hypokalemia and hypomagnesemia increase the risk of QT prolongation. Hypokalemia can result from diuretic therapy, diarrhea, and other causes. Use caution when treating patients at risk for significant electrolyte disturbance, particularly hypokalemia. Obtain baseline measurements of serum potassium and magnesium levels, and periodically monitor electrolytes. Correct electrolyte abnormalities before initiating treatment with clozapine.
- DRUG INTERACTIONS
- Drugs that Cause QT Interval Prolongation
- Use caution when administering concomitant medications that prolong the QT interval or inhibit the metabolism of clozapine. Drugs that cause QT prolongation include: specific antipsychotics (e.g., ziprasidone, iloperidone, chlorpromazine, thioridazine, mesoridazine, droperidol, and pimozide), specific antibiotics (e.g., erythromycin, gatifloxacin, moxifloxacin, sparfloxacin), Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class III antiarrhythmics (e.g., amiodarone, sotalol), and others (e.g., pentamidine, levomethadyl acetate, methadone, halofantrine, mefloquine, dolasetron mesylate, probucol or tacrolimus) [see WARNINGS AND PRECAUTIONS (5.10)].
- ADVERSE REACTIONS
- Postmarketing Experience
- Cardiovascular System
- Atrial or ventricular fibrillation, ventricular tachycardia, palpitations, QT interval prolongation, Torsades de Pointes, mitral valve incompetence associated with clozapine-related cardiomyopathy, myocardial infarction sometimes fatal, cardiac arrest, myocarditis sometimes fatal and periorbital edema.
- PATIENT COUNSELING INFORMATION
- QT Interval Prolongation: Advise patients to consult their clinician immediately if they feel faint, lose consciousness or have signs or symptoms suggestive of arrhythmia. Instruct patients to not take clozapine with other drugs that cause QT interval prolongation. Instruct patients to inform their clinicians that they are taking clozapine before any new drug [see WARNINGS AND PRECAUTIONS (5.10), DRUG INTERACTIONS (7.1)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
220
42692
Other ADRs
58776
14058503
Odds Ratio = 1.233
Drug Property Information
ATC Code(s):
- N05AH02 - clozapine
- N05AH - "Diazepines, oxazepines and thiazepines"
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:clozapine
Active Ingredient UNII:J60AR2IKIC
Drugbank ID:DB00363
PubChem Compound:2818
CAS Number:5786-21-0
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day N05AH02
Chemical Structure: 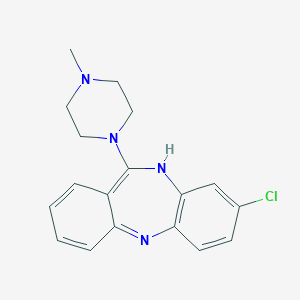

SMILE Code:
CN1CCN(CC1)C2=C3C=CC=CC3=NC4=C(N2)C=C(C=C4)Cl
CN1CCN(CC1)C2=C3C=CC=CC3=NC4=C(N2)C=C(C=C4)Cl
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rhabdomyolysis in Clozapine Overdose.
[Jansman Frank G A,Crommelin Heleen A,van Hout Freek J A H,Meulenbelt Jan]Drug Saf Case Rep.2015 Dec;2(1):9. PMID: 27747721
2: Recurrent rhabdomyolysis secondary to hyponatremia in a patient with primary psychogenic polydipsia.
[Aguiar Diana Tavares,Monteiro Catarina,Coutinho Paula]Rev Bras Ter Intensiva.2015 Jan-Mar;27(1):77-81. doi: 10.5935/0103-507X.20150013. Epub 2015 Mar 1. PMID: 25909317
3: Electroconvulsive in a Schizophrenic Patient With Neuroleptic Malignant Syndrome and Rhabdomyolysis.
[San Gabriel Maria Chona P,Eddula-Changala Bharathi,Tan Yonghong,Longshore Carrol T]J ECT.2015 Sep;31(3):197-200. doi: 10.1097/YCT.0000000000000184. PMID: 25243752
4: Rhabdomyolysis reported for children and adolescents treated with antipsychotic medicines: a case series analysis.
[Star Kristina,Iessa Noha,Almandil Noor B,Wilton Lynda,Curran Sarah,Edwards I Ralph,Wong Ian C K]J Child Adolesc Psychopharmacol.2012 Dec;22(6):440-51. doi: 10.1089/cap.2011.0134. PMID: 23234587
5: Neuroleptic malignant syndrome secondary to aripiprazole initiation in a clozapine-intolerant patient.
[Patel Mitesh K,Brunetti Luigi]Am J Health Syst Pharm.2010 Aug;67(15):1254-9. doi: 10.2146/ajhp090243. PMID: 20651315
6: Rhabdomyolysis following dose increase of clozapine and combination therapy with lithium.
[Tseng Kuan-Chiao,Hwang Tzung-Jeng]J Clin Psychopharmacol.2009 Aug;29(4):398-9. doi: 10.1097/JCP.0b013e3181accfc3. PMID: 19593187
7: Ciprofloxacin strongly inhibits clozapine metabolism: two case reports.
[Brouwers E E M,Söhne M,Kuipers S,van Gorp E C M,Schellens J H M,Koks C H W,Beijnen J H,Huitema A D R]Clin Drug Investig.2009;29(1):59-63. doi: 10.2165/0044011-200929010-00006. PMID: 19067475
8: Fatal toxicity of drugs used in psychiatry.
[Flanagan Robert J]Hum Psychopharmacol.2008 Jan;23 Suppl 1:43-51. PMID: 18098225
9: Successful switch to olanzapine after rhabdomyolysis caused by water intoxication and clozapine use.
[Tényi T,Vörös V]Pharmacopsychiatry.2006 Jul;39(4):157-8. PMID: 16871473
10: [Neuroleptic malignant syndrome after 30 years treatment with clozapine: a rarely seen differential diagnosis on intensive care units].
[Franzen D,Burkhard J,Corti N,Schüpbach D,Fontanel D,Stäubli M]Anasthesiol Intensivmed Notfallmed Schmerzther.2006 Feb;41(2):125-7. PMID: 16493563
11: Rhabdomyolysis after correction of hyponatremia in psychogenic polydipsia possibly complicated by ziprasidone.
[Zaidi Ali N]Ann Pharmacother.2005 Oct;39(10):1726-31. Epub 2005 Aug 30. PMID: 16131536
12: Neuroleptic malignant syndrome in an adolescent after brief exposure to olanzapine.
[Hanft Alan,Eggleston Christopher F,Bourgeois James A]J Child Adolesc Psychopharmacol.2004 Fall;14(3):481-7. PMID: 15650507
13: Malignant McLeod myopathy.
[Jung Hans H,Brandner Sebastian]Muscle Nerve.2002 Sep;26(3):424-7. PMID: 12210375
14: Monitoring of clozapine and norclozapine plasma concentration-time curves in acute overdose.
[Renwick A C,Renwick A G,Flanagan R J,Ferner R E]J Toxicol Clin Toxicol.2000;38(3):325-8. PMID: 10866334
15: Fatal status epilepticus associated with olanzapine therapy.
[Wyderski R J,Starrett W G,Abou-Saif A]Ann Pharmacother.1999 Jul-Aug;33(7-8):787-9. PMID: 10466904
16: Rhabdomyolysis after correction of hyponatremia due to psychogenic polydipsia possibly complicated by clozapine.
[Wicki J,Rutschmann O T,Burri H,Vecchietti G,Desmeules J]Ann Pharmacother.1998 Sep;32(9):892-5. PMID: 9762377
17: Rhabdomyolysis associated with clozapine treatment in a patient with decreased calcium-dependent potassium permeability of cell membranes.
[Koren W,Koren E,Nacasch N,Ehrenfeld M,Gur H]Clin Neuropharmacol.1998 Jul-Aug;21(4):262-4. PMID: 9704170
18: Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment.
[Meltzer H Y,Cola P A,Parsa M]Neuropsychopharmacology.1996 Oct;15(4):395-405. PMID: 8887994
19: [A high serum creatine kinase picture due to too much or too little exercise].
[Weber M,Gerber H]Dtsch Med Wochenschr.1995 Jun 23;120(25-26):917-20. PMID: 7600928
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.