Search for drugs:
Typing the drug name to query
ARIPIPRAZOLE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Human Experience
- In clinical trials and in postmarketing experience, adverse reactions of deliberate or accidental overdosage with oral aripiprazole have been reported worldwide. These include overdoses with oral aripiprazole alone and in combination with other substances. No fatality was reported with aripiprazole alone. The largest known dose with a known outcome involved acute ingestion of 1260 mg of oral aripiprazole (42 times the maximum recommended daily dose) by a patient who fully recovered. Deliberate or accidental overdosage was also reported in children (age 12 and younger) involving oral aripiprazole ingestions up to 195 mg with no fatalities.
- Common adverse reactions (reported in at least 5% of all overdose cases) reported with oral aripiprazole overdosage (alone or in combination with other substances) include vomiting, somnolence, and tremor. Other clinically important signs and symptoms observed in one or more patients with aripiprazole overdoses (alone or with other substances) include acidosis, aggression, aspartate aminotransferase increased, atrial fibrillation, bradycardia, coma, confusional state, convulsion, blood creatine phosphokinase increased, depressed level of consciousness, hypertension, hypokalemia, hypotension, lethargy, loss of consciousness, QRS complex prolonged, QT prolonged, pneumonia aspiration, respiratory arrest, status epilepticus, and tachycardia.
- [Management of Overdosage]
- No specific information is available on the treatment of overdose with aripiprazole. An electrocardiogram should be obtained in case of overdosage and if QT interval prolongation is present, cardiac monitoring should be instituted. Otherwise, management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Close medical supervision and monitoring should continue until the patient recovers.
- Charcoal: In the event of an overdose of aripiprazole, an early charcoal administration may be useful in partially preventing the absorption of aripiprazole. Administration of 50 g of activated charcoal, one hour after a single 15 mg oral dose of aripiprazole, decreased the mean AUC and C max of aripiprazole by 50%.
- ADVERSE REACTIONS
- Clinical Trials Experience
- Investigations:
- frequent - weight decreased, infrequent - hepatic enzyme increased, blood glucose increased, blood lactate dehydrogenase increased, gamma glutamyl transferase increased; rare – blood prolactin increased, blood urea increased, blood creatinine increased, blood bilirubin increased, electrocardiogram QT prolonged, glycosylated hemoglobin increased
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
170
42742
Other ADRs
38547
14078732
Odds Ratio = 1.453
Drug Property Information
ATC Code(s):
- N05AX12 - aripiprazole
- N05AX - Other antipsychotics
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:aripiprazole
Active Ingredient UNII:82VFR53I78
Drugbank ID:DB01238
PubChem Compound:60795
CAS Number:129722-12-9
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 15.0 mg/day N05AX12
Chemical Structure: 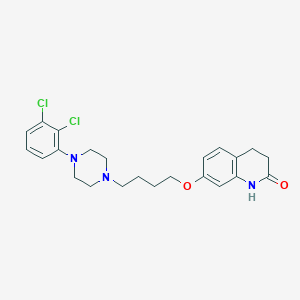

SMILE Code:
C1CC(=O)NC2=C1C=CC(=C2)OCCCCN3CCN(CC3)C4=C(C(=CC=C4)Cl)Cl
C1CC(=O)NC2=C1C=CC(=C2)OCCCCN3CCN(CC3)C4=C(C(=CC=C4)Cl)Cl
Reference
COHORT STUDY:
1: A Retrospective Cohort Study of Acute Kidney Injury Risk Associated with Antipsychotics.
[Jiang Y, McCombs JS, Park SH, CNS Drugs. 2017 Apr;31(4):319-326.]ABSTRACT
BACKGROUND: A recent large database analysis raised concerns of potential acute kidney injury (AKI) risk associated with antipsychotics. However, whether individual atypical and typical antipsychotics are associated with differential AKI risks has not been investigated.
OBJECTIVE: The current study compared the risks of AKI and known causes of AKI associated with a broad range of atypical and typical antipsychotics.
METHOD: This retrospective cohort analysis used January 2007-June 2013 US nationwide Humana claims data to define episodes of antipsychotic drug therapy for patients with schizophrenia and bipolar disorder. Study drugs were aripiprazole, fluphenazine, haloperidol, olanzapine, quetiapine, risperidone, and ziprasidone. Study outcomes were hospitalizations with AKI, and known causes of AKI, i.e., hypotension, acute urinary retention, neuroleptic malignant syndrome/rhabdomyolysis, and pneumonia. AKI was the primary outcome of the study. Cox regressions using haloperidol as the baseline comparator were used to estimate the impact of alternative antipsychotics on the risks of study adverse events following the initiation of treatment. The Cox models controlled for treatment history, comorbidities, and concomitant drug use in the prior 6 months. They also controlled for patient demographics and dose of current treatment.
RESULTS: The overall incidence of AKI was 25.0 per 1000 person-years. According to our multivariate regression results, the risk of AKI was significantly increased in patients taking olanzapine [hazard ratio (HR) 1.344, 95% confidence interval (CI) 1.057-1.708], quetiapine (HR 1.350, 95% CI 1.082-1.685), and ziprasidone (HR 1.338, 95% CI 1.035-1.729) relative to haloperidol. Aripiprazole (HR 1.152, 95% CI 0.908-1.462) and risperidone (HR 1.147, 95% CI 0.923-1.426) had insignificantly higher risks of AKI compared with haloperidol, whereas fluphenazine (HR 0.729, 95% CI 0.483-1.102) had an insignificantly lower risk of AKI. When compared between drug classes, atypical antipsychotics had a significantly higher risk of AKI (HR 1.313, 95% CI 1.083-1.591) than typical antipsychotics.
CONCLUSIONS: Antipsychotics are associated with differential AKI risks, with several atypical antipsychotics having higher risks than haloperidol. However, the overall incidence of AKI was moderate, and AKI risk should only raise concern for clinicians with elderly patients or patients who are vulnerable to kidney disease.
PMID: 28290080
OBJECTIVE: The current study compared the risks of AKI and known causes of AKI associated with a broad range of atypical and typical antipsychotics.
METHOD: This retrospective cohort analysis used January 2007-June 2013 US nationwide Humana claims data to define episodes of antipsychotic drug therapy for patients with schizophrenia and bipolar disorder. Study drugs were aripiprazole, fluphenazine, haloperidol, olanzapine, quetiapine, risperidone, and ziprasidone. Study outcomes were hospitalizations with AKI, and known causes of AKI, i.e., hypotension, acute urinary retention, neuroleptic malignant syndrome/rhabdomyolysis, and pneumonia. AKI was the primary outcome of the study. Cox regressions using haloperidol as the baseline comparator were used to estimate the impact of alternative antipsychotics on the risks of study adverse events following the initiation of treatment. The Cox models controlled for treatment history, comorbidities, and concomitant drug use in the prior 6 months. They also controlled for patient demographics and dose of current treatment.
RESULTS: The overall incidence of AKI was 25.0 per 1000 person-years. According to our multivariate regression results, the risk of AKI was significantly increased in patients taking olanzapine [hazard ratio (HR) 1.344, 95% confidence interval (CI) 1.057-1.708], quetiapine (HR 1.350, 95% CI 1.082-1.685), and ziprasidone (HR 1.338, 95% CI 1.035-1.729) relative to haloperidol. Aripiprazole (HR 1.152, 95% CI 0.908-1.462) and risperidone (HR 1.147, 95% CI 0.923-1.426) had insignificantly higher risks of AKI compared with haloperidol, whereas fluphenazine (HR 0.729, 95% CI 0.483-1.102) had an insignificantly lower risk of AKI. When compared between drug classes, atypical antipsychotics had a significantly higher risk of AKI (HR 1.313, 95% CI 1.083-1.591) than typical antipsychotics.
CONCLUSIONS: Antipsychotics are associated with differential AKI risks, with several atypical antipsychotics having higher risks than haloperidol. However, the overall incidence of AKI was moderate, and AKI risk should only raise concern for clinicians with elderly patients or patients who are vulnerable to kidney disease.
OTHER REFERENCE(S):
1: Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: A case report.
[Zhu Xiuqing,Hu Jinqing,Deng Shuhua,Qiu Chang,Shang Dewei,Wen Yuguan]Aust N Z J Psychiatry.2018 Feb;52(2):206. doi: 10.1177/0004867417743342. Epub 2017 Dec 14. PMID: 29241352
2: A Retrospective Cohort Study of Acute Kidney Injury Risk Associated with Antipsychotics.
[Jiang Yawen,McCombs Jeffrey S,Park Susie H]CNS Drugs.2017 Apr;31(4):319-326. doi: 10.1007/s40263-017-0421-4. PMID: 28290080
3: Factors Affecting the Timing of Signal Detection of Adverse Drug Reactions.
[Hashiguchi Masayuki,Imai Shungo,Uehara Keiko,Maruyama Junya,Shimizu Mikiko,Mochizuki Mayumi]PLoS One.2015 Dec 7;10(12):e0144263. doi: 10.1371/journal.pone.0144263. eCollection 2015. PMID: 26641634
4: Rhabdomyolysis reported for children and adolescents treated with antipsychotic medicines: a case series analysis.
[Star Kristina,Iessa Noha,Almandil Noor B,Wilton Lynda,Curran Sarah,Edwards I Ralph,Wong Ian C K]J Child Adolesc Psychopharmacol.2012 Dec;22(6):440-51. doi: 10.1089/cap.2011.0134. PMID: 23234587
5: Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation.
[Marzetti Emanuele,Bocchino Lorenzo,Teramo Stefano,Scudieri Giorgio,Aulisa Angelo Gabriele]J Neuropsychiatry Clin Neurosci.2012 Fall;24(4):E40-1. doi: 10.1176/appi.neuropsych.11110328. PMID: 23224480
6: Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia.
[Wu Yung-Fu,Chang Kuo-Yung]J Neuropsychiatry Clin Neurosci.2011 Summer;23(3):E51. doi: 10.1176/appi.neuropsych.23.3.E51. PMID: 21948930
7: Psychotropic drug-related eosinophilia with systemic symptoms after acute caffeine ingestion.
[Mahapatra Sidharth,Belgrad Jonathan L,Adeoye Martins A]Pediatrics.2011 Jan;127(1):e235-8. doi: 10.1542/peds.2010-0374. Epub 2010 Dec 6. PMID: 21135003
8: Neuroleptic malignant syndrome secondary to aripiprazole initiation in a clozapine-intolerant patient.
[Patel Mitesh K,Brunetti Luigi]Am J Health Syst Pharm.2010 Aug;67(15):1254-9. doi: 10.2146/ajhp090243. PMID: 20651315
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.