Search for drugs:
Typing the drug name to query
LAMIVUDINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- In addition to adverse reactions reported from clinical trials, the following adverse reactions have been reported during postmarketing use of lamivudine. Because these reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to lamivudine.
- Body as a Whole: Redistribution/accumulation of body fat [see WARNINGS AND PRECAUTIONS (5.7)].
- Endocrine and Metabolic: Hyperglycemia.
- General: Weakness.
- Hemic and Lymphatic: Anemia (including pure red cell aplasia and severe anemias progressing on therapy).
- Hepatic and Pancreatic: Lactic acidosis and hepatic steatosis, posttreatment exacerbation of hepatitis B [see BOXED WARNING, Warnings and Precautions (5.1, 5.2)].
- Hypersensitivity: Anaphylaxis, urticaria.
- Musculoskeletal: Muscle weakness, CPK elevation, rhabdomyolysis.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
58
42854
Other ADRs
9321
14107958
Odds Ratio = 2.049
Drug Property Information
ATC Code(s):
- J05AR02 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR13 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR12 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR01 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR05 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR07 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR04 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AF05 - lamivudine
- J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR16 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR11 - lamivudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:lamivudine
Active Ingredient UNII:2T8Q726O95
Drugbank ID:DB00709
PubChem Compound:60825
CAS Number:134678-17-4
Dosage Form(s):solution
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day J05AF05
Chemical Structure: 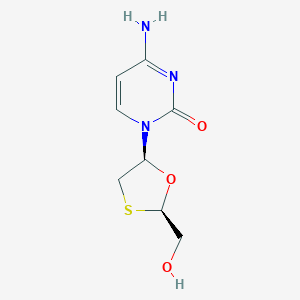

SMILE Code:
C1[C@H](O[C@H](S1)CO)N2C=CC(=NC2=O)N
C1[C@H](O[C@H](S1)CO)N2C=CC(=NC2=O)N
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rhabdomyolysis due to lamivudine re-administration in a patient with HBV-related hepatic failure caused by interruption of lamivudine and adefovir.
[Kim Soo Ki,Kim Soo Ryang,Imoto Susumu,Tohyama Madoka,Otono Yumi,Tamura Tomoko]J Gastrointestin Liver Dis.2015 Dec;24(4):535-6. PMID: 26697584
2: Rhabdomyolysis due to Lamivudine administration in acute viral hepatitis B infection: a case report from Malaysia.
[Baharin Janudin,Sahari Narisa Sulaiman,Lim Sazlyna Mohd Sazlly]Electron Physician.2014 Jul 1;6(3):863-7. doi: 10.14661/2014.863-867. eCollection 2014 Jul-Sep. PMID: 25763159
3: Tolerability of HIV postexposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir tablet formulation.
[Tosini William,Muller Philippe,Prazuck Thierry,Benabdelmoumen Ghania,Peyrouse Eric,Christian Bernard,Quertainmont Yann,Bouvet Elisabeth,Rabaud Christian]AIDS.2010 Sep 24;24(15):2375-80. doi: 10.1097/QAD.0b013e32833dfad1. PMID: 20729709
4: Severe myositis on commencement of efavirenz, abacavir and lamivudine, in the absence of lactic acidosis or classical abacavir hypersensitivity.
[Parsonage Mirella Jane,Barlow Gavin,Lillie Patrick,Moss Peter,Adams Katherine,Thaker Hiten]BMJ Case Rep.2009;2009. pii: bcr01.2009.1411. doi: 10.1136/bcr.01.2009.1411. Epub 2009 Jun 15. PMID: 21687032
5: Rhabdomyolysis due to Lamivudine administration in a liver transplant recipient.
[Adani Gian Luigi,Baccarani Umberto,Risaliti Andrea,Bresadola Fabrizio,Della Rocca Giorgio,Viale Pierluigi]Am J Transplant.2005 Mar;5(3):634. PMID: 15707423
6: Anti-retrovirals and immunosuppressive drug interactions in a HIV-positive patient after liver transplantation.
[Antonini Mario,Ettorre Giuseppe Maria,Vennarecci Giovanni,D'Offizi Gianpiero,Narciso Pasquale,Del Nonno Franca,Perracchio Letizia,Visco Giuseppe,Santoro Eugenio]Hepatogastroenterology.2004 May-Jun;51(57):646-8. PMID: 15143883
7: [Severe lactic acidosis in HIV-infected patients treated with nucleosidic reverse transcriptase analogs: a report of 9 cases].
[Bonnet F,Bonarek M,Abridj A,Mercié P,Dupon M,Gemain M-C,Malvy D,Bernard N,Pellegrin J-L,Morlat P,Beylot J]Rev Med Interne.2003 Jan;24(1):11-6. PMID: 12614853
8: Rhabdomyolytic syndrome during the lamivudine therapy for acute exacerbation of chronic type B hepatitis.
[Yahagi Kaichiro,Ueno Yoshiyuki,Mano Yutaka,Shimosegawa Tooru]Liver Transpl.2002 Dec;8(12):1198-9. PMID: 12474162
9: From the Centers for Disease Control and Prevention. Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, 1997-2000.
JAMA.2001 Jan 24-31;285(4):402-3. PMID: 11263401
10: Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, 1997-2000.
[Centers for Disease Control and Prevention (CDC)]MMWR Morb Mortal Wkly Rep.2001 Jan 5;49(51-52):1153-6. PMID: 11198946
11: [Rhabdomyolysis in antiretroviral therapy with Lamivudin].
[Mendila M,Walter G F,Stoll M,Schmidt R E]Dtsch Med Wochenschr.1997 Aug 15;122(33):1003-6. PMID: 9296927
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.