Search for drugs:
Typing the drug name to query
FOSAMPRENAVIR CALCIUM
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
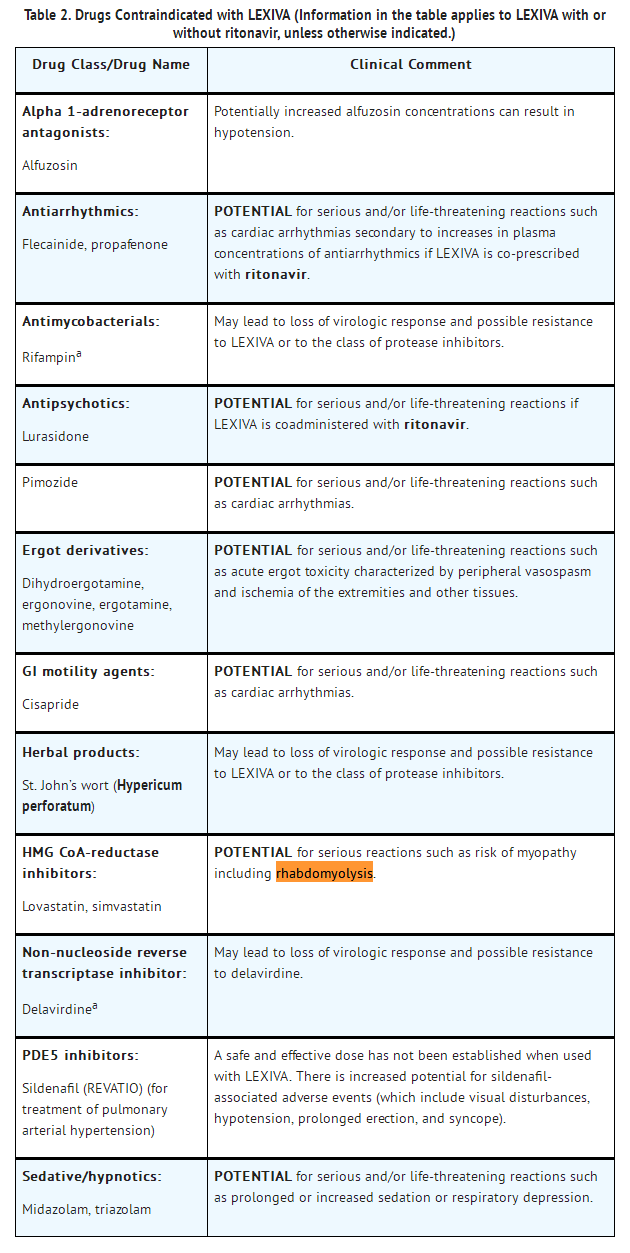
- CONTRAINDICATIONS
- LEXIVA is contraindicated:
- • in patients with previously demonstrated clinically significant hypersensitivity (e.g., Stevens-Johnson syndrome) to any of the components of this product or to amprenavir.
- • when coadministered with drugs that are highly dependent on cytochrome P450 3A4 (CYP3A4) for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events ( TABLE 2).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
10
42902
Other ADRs
662
14116617
Odds Ratio = 4.971
Drug Property Information
ATC Code(s):
- J05AE07 - fosamprenavir calcium
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:fosamprenavir calcium
Active Ingredient UNII:ID1GU2627N
Drugbank ID:DB01319
PubChem Compound:131536
CAS Number:226700-79-4
Dosage Form(s):suspension; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 1400.0 mg/day J05AE07
Chemical Structure: 

SMILE Code:
CC(C)CN(C[C@H]([C@H](CC1=CC=CC=C1)NC(=O)O[C@H]2CCOC2)OP(=O)(O)O)S(=O)(=O)C3=CC=C(C=C3)N
CC(C)CN(C[C@H]([C@H](CC1=CC=CC=C1)NC(=O)O[C@H]2CCOC2)OP(=O)(O)O)S(=O)(=O)C3=CC=C(C=C3)N
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.