Search for drugs:
Typing the drug name to query
QUININE SULFATE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Use of Quinine Sulfate for Treatment or Prevention of Nocturnal Leg Cramps
- Quinine sulfate may cause unpredictable serious and life-threatening hematologic reactions including thrombocytopenia and hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP) in addition to hypersensitivity reactions, QT prolongation, serious cardiac arrhythmias including torsades de pointes, and other serious adverse events requiring medical intervention and hospitalization. Chronic renal impairment associated with the development of TTP, and fatalities have also been reported. The risk associated with the use of quinine sulfate in the absence of evidence of its effectiveness for treatment or prevention of nocturnal leg cramps, outweighs any potential benefit in treating and/or preventing this benign, self-limiting condition [see BOXED WARNING and Contraindications (4)].
- [QT Prolongation and Ventricular Arrhythmias]
- QT interval prolongation has been a consistent finding in studies which evaluated electrocardiographic changes with oral or parenteral quinine administration, regardless of age, clinical status, or severity of disease. The maximum increase in QT interval has been shown to correspond with peak quinine plasma concentration [see Clinical Pharmacology (12.2)]. Quinine sulfate has been rarely associated with potentially fatal cardiac arrhythmias, including torsades de pointes, and ventricular fibrillation.
- Quinine sulfate has been shown to cause concentration-dependent prolongation of the PR and QRS interval. At particular risk are patients with underlying structural heart disease and preexisting conduction system abnormalities, elderly patients with sick sinus syndrome, patients with atrial fibrillation with slow ventricular response, patients with myocardial ischemia or patients receiving drugs known to prolong the PR interval (e.g., verapamil) or QRS interval (e.g., flecainide or quinidine) [see Clinical Pharmacology (12.2)].
- Quinine sulfate is not recommended for use with other drugs known to cause QT prolongation, including Class IA antiarrhythmic agents (e.g., quinidine, procainamide, disopyramide), and Class III antiarrhythmic agents (e.g., amiodarone, sotalol, dofetilide).
- The use of macrolide antibiotics such as erythromycin should be avoided in patients receiving quinine sulfate. Fatal torsades de pointes was reported in an elderly patient who received concomitant quinine, erythromycin, and dopamine. Although a causal relationship between a specific drug and the arrhythmia was not established in this case, erythromycin is a CYP3A4 inhibitor and has been shown to increase quinine plasma levels when used concomitantly. A related macrolide antibiotic, troleandomycin, has also been shown to increase quinine exposure in a pharmacokinetic study [see Drug Interactions (7)].
- Quinine may inhibit the metabolism of certain drugs that are CYP3A4 substrates and are known to cause QT prolongation, e.g., astemizole, cisapride, terfenadine, pimozide, halofantrine and quinidine. Torsades de pointes has been reported in patients who received concomitant quinine and astemizole. Therefore, concurrent use of quinine sulfate with these medications, or drugs with similar properties, should be avoided [see Drug Interactions (7)].
- Concomitant administration of quinine sulfate with the antimalarial drugs, mefloquine or halofantrine, may result in electrocardiographic abnormalities, including QT prolongation, and increase the risk for torsades de pointes or other serious ventricular arrhythmias. Concurrent use of quinine sulfate and mefloquine may also increase the risk of seizures [see Drug Interactions (7)].
- Quinine sulfate should also be avoided in patients with known prolongation of QT interval and in patients with clinical conditions known to prolong the QT interval, such as uncorrected hypokalemia, bradycardia, and certain cardiac conditions [see Contraindications (4)].
- DRUG INTERACTIONS
- figure
- CONTRAINDICATIONS
- Quinine sulfate capsules are contraindicated in patients with the following:
- Prolonged QT interval. One case of a fatal ventricular arrhythmia was reported in an elderly patient with a prolonged QT interval at baseline, who received quinine sulfate intravenously for P. falciparum malaria [see Warnings and Precautions (5.4)].
- OVERDOSAGE
- Quinine, like quinidine, has Class I antiarrhythmic properties. The cardiotoxicity of quinine is due to its negative inotropic action, and to its effect on cardiac conduction, resulting in decreased rates of depolarization and conduction, and increased action potential and effective refractory period. ECG changes observed with quinine overdose include sinus tachycardia, PR prolongation, T wave inversion, bundle branch block, an increased QT interval, and a widening of the QRS complex. Quinine’s alpha-blocking properties may result in hypotension and further exacerbate myocardial depression by decreasing coronary perfusion. Quinine overdose has been also associated with hypotension, cardiogenic shock, and circulatory collapse, ventricular arrhythmias, including ventricular tachycardia, ventricular fibrillation, idioventricular rhythm, and torsades de pointes, as well as bradycardia, and atrioventricular block [see Warnings and Precautions (5) and Clinical Pharmacology (12.3)].
- ADVERSE REACTIONS
- Cardiovascular: chest pain, vasodilatation, hypotension, postural hypotension, tachycardia, bradycardia, palpitations, syncope, atrioventricular block, atrial fibrillation, irregular rhythm, unifocal premature ventricular contractions, nodal escape beats, U waves, QT prolongation, ventricular fibrillation, ventricular tachycardia, torsades de pointes, and cardiac arrest.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- QTc interval prolongation was studied in a double-blind, multiple dose, placebo- and positive-controlled crossover study in young (N=13, 20 to 39 years) and elderly (N=13, 65 to 78 years) subjects. After 7 days of dosing with quinine sulfate 648 mg three times daily, the maximum mean (95% upper confidence bound) differences in QTcI from placebo after baseline correction was 27.7 (32.2) ms.
- [Pharmacokinetics]
- Effects of quinine on other drugs
- Mefloquine: In 7 healthy subjects who received mefloquine (750 mg) at 24 hours before an oral 600 mg dose of quinine sulfate, the AUC of mefloquine was increased by 22% compared to mefloquine alone. In this study, the QTc interval was significantly prolonged in the subjects who received mefloquine and quinine sulfate 24 hours apart [see Drug Interactions (7)].
- MEDICATION GUIDE
- Do not take quinine sulfate capsules if you have:
- changes in the electrical activity of your heart called QT prolongation
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
2
42910
Other ADRs
593
14116686
Odds Ratio = 1.11
Drug Property Information
ATC Code(s):
- M09AA72 - quinine sulfate
- M09AA - Quinine and derivatives
- M09A - OTHER DRUGS FOR DISORDERS OF THE MUSCULO-SKELETAL SYSTEM
- M09 - OTHER DRUGS FOR DISORDERS OF THE MUSCULO-SKELETAL SYSTEM
- M - MUSCULO-SKELETAL SYSTEM
- P01BC01 - quinine sulfate
- P01BC - Methanolquinolines
- P01B - ANTIMALARIALS
- P01 - ANTIPROTOZOALS
- P - "ANTIPARASITIC PRODUCTS, INSECTICIDES AND REPELLENTS"
Active Ingredient:quinine sulfate
Active Ingredient UNII:KF7Z0E0Q2B
Drugbank ID:DB00468
PubChem Compound:3034034
CAS Number:130-95-0
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 1500.0 mg/day P01BC01
Chemical Structure: 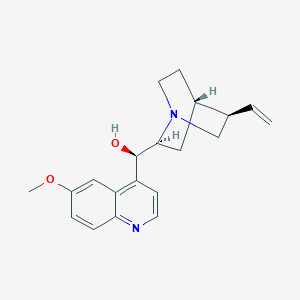

SMILE Code:
COC1=CC2=C(C=CN=C2C=C1)[C@H]([C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)O
COC1=CC2=C(C=CN=C2C=C1)[C@H]([C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.