Search for drugs:
Typing the drug name to query
ATOMOXETINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Human Experience
- There is limited clinical trial experience with atomoxetine overdose. During postmarketing, there have been fatalities reported involving a mixed ingestion overdose of atomoxetine and at least one other drug. There have been no reports of death involving overdose of atomoxetine alone, including intentional overdoses at amounts up to 1400 mg. In some cases of overdose involving atomoxetine, seizures have been reported. The most commonly reported symptoms accompanying acute and chronic overdoses of atomoxetine were gastrointestinal symptoms, somnolence, dizziness, tremor, and abnormal behavior. Hyperactivity and agitation have also been reported. Signs and symptoms consistent with mild to moderate sympathetic nervous system activation (e.g., tachycardia, blood pressure increased, mydriasis, dry mouth) have also been observed. Most events were mild to moderate. Less commonly, there have been reports of QT prolongation and mental changes, including disorientation and hallucinations [see CLINICAL PHARMACOLOGY (12.2)].
- ADVERSE REACTIONS
- Postmarketing Spontaneous Reports
- The following adverse reactions have been identified during post approval use of atomoxetine. Unless otherwise specified, these adverse reactions have occurred in adults and children and adolescents. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular system – QT prolongation, syncope.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- An exposure-response analysis encompassing doses of atomoxetine (0.5, 1.2 or 1.8 mg/kg/day) or placebo demonstrated atomoxetine exposure correlates with efficacy as measured by the Attention-Deficit/Hyperactivity Disorder Rating Scale-IV-Parent Version: Investigator administered and scored. The exposure-efficacy relationship was similar to that observed between dose and efficacy with median exposures at the two highest doses resulting in near maximal changes from baseline [see CLINICAL STUDIES (14.2)].
- Cardiac Electrophysiology – The effect of atomoxetine on QTc prolongation was evaluated in a randomized, double-blinded, positive-(moxifloxacin 400 mg) and placebo-controlled, cross-over study in healthy male CYP2D6 poor metabolizers. A total of 120 healthy subjects were administered atomoxetine (20 mg and 60 mg) twice daily for 7 days. No large changes in QTc interval (i.e., increases >60 msec from baseline, absolute QTc >480 msec) were observed in the study. However, small changes in QTc interval cannot be excluded from the current study, because the study failed to demonstrate assay sensitivity. There was a slight increase in QTc interval with increased atomoxetine concentration.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
13
42899
Other ADRs
21805
14095474
Odds Ratio = 0.196
Drug Property Information
ATC Code(s):
- N06BA09 - atomoxetine
- N06BA - Centrally acting sympathomimetics
- N06B - "PSYCHOSTIMULANTS, AGENTS USED FOR ADHD AND NOOTROPICS"
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:atomoxetine hydrochloride
Active Ingredient UNII:57WVB6I2W0
Drugbank ID:DB00289
PubChem Compound:54841
CAS Number:83015-26-3
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 80.0 mg/day N06BA09
Chemical Structure: 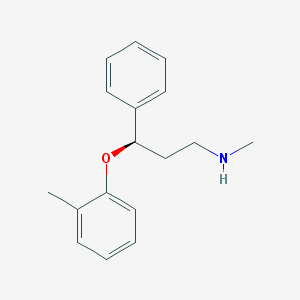

SMILE Code:
CC1=CC=CC=C1O[C@H](CCNC)C2=CC=CC=C2
CC1=CC=CC=C1O[C@H](CCNC)C2=CC=CC=C2
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [Pharmacovigilance update].
[Livio F]Rev Med Suisse.2013 Jan 9;9(368):72-5. PMID: 23367709
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.