Search for drugs:
Typing the drug name to query
LETERMOVIR
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
- >>1b49df80-be4f-47e0-a0b7-123f3e69395b.jpeg
- CONTRAINDICATIONS
- PREVYMIS is contraindicated in patients receiving pimozide or ergot alkaloids:
- Pimozide: Concomitant administration of PREVYMIS in patients receiving pimozide may result in increased concentrations of pimozide due to inhibition of cytochrome P450 3A (CYP3A) by letermovir, which may lead to QT prolongation and torsades de pointes [see WARNINGS AND PRECAUTIONS (5.1) and DRUG INTERACTIONS (7.2, 7.3)].
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a thorough QT trial in healthy subjects, letermovir at the therapeutic IV dose or at a dose of 2 times the approved IV dose did not prolong QTc to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
0
14117279
Odds Ratio = N/A
Drug Property Information
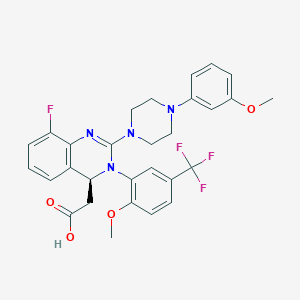
SMILE Code:
COC1=C(C=C(C=C1)C(F)(F)F)N2[C@H](C3=C(C(=CC=C3)F)N=C2N4CCN(CC4)C5=CC(=CC=C5)OC)CC(=O)O
COC1=C(C=C(C=C1)C(F)(F)F)N2[C@H](C3=C(C(=CC=C3)F)N=C2N4CCN(CC4)C5=CC(=CC=C5)OC)CC(=O)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.