Search for drugs:
Typing the drug name to query
MORPHINE SULFATE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Acute overdosage with morphine can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, rhabdomyolysis progressing to renal failure, and, sometimes, bradycardia, hypotension and death.
- The nature of the controlled-release morphine should also be taken into account when treating the overdose. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects. Deaths due to overdose may occur with abuse and misuse of morphine sulfate extended-release tablets.
- In the treatment of morphine overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen, vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
- The pure opioid antagonists, such as naloxone, are specific antidotes against respiratory depression which results from opioid overdose. Naloxone (usually should be administered intravenously; however, because its duration of action is relatively short, the patient must be carefully monitored until spontaneous respiration is reliably re-established. If the response to naloxone is suboptimal or not sustained, additional naloxone may be administered, as needed, or given by continuous infusion to maintain alertness and respiratory function; however, there is no information available about the cumulative dose of naloxone that may be safely administered.
- Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to morphine overdose. Such agents should be administered cautiously to persons who are known, or suspected to be physically dependent on morphine sulfate extended-release tablets. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
130
42782
Other ADRs
22246
14095033
Odds Ratio = 1.926
Drug Property Information
ATC Code(s):
- N02AA01 - morphine sulfate
- N02AA - Natural opium alkaloids
- N02A - OPIOIDS
- N02 - ANALGESICS
- N - NERVOUS SYSTEM
- A07DA52 - morphine sulfate
- A07DA - Antipropulsives
- A07D - ANTIPROPULSIVES
- A07 - "ANTIDIARRHEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVE "
- A - ALIMENTARY TRACT AND METABOLISM
- N02AA51 - morphine sulfate
- N02AA - Natural opium alkaloids
- N02A - OPIOIDS
- N02 - ANALGESICS
- N - NERVOUS SYSTEM
- N02AG01 - morphine sulfate
- N02AG - Opioids in combination with antispasmodics
- N02A - OPIOIDS
- N02 - ANALGESICS
- N - NERVOUS SYSTEM
Active Ingredient:morphine sulfate
Active Ingredient UNII:X3P646A2J0
Drugbank ID:DB00295
PubChem Compound:5288826
CAS Number:57-27-2
Dosage Form(s):tablet, film coated, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 100.0 mg/day N02AA01
Chemical Structure: 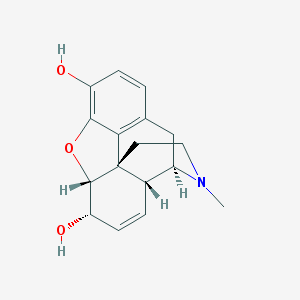

SMILE Code:
CN1CC[C@]23[C@@H]4[C@H]1CC5=C2C(=C(C=C5)O)O[C@H]3[C@H](C=C4)O
CN1CC[C@]23[C@@H]4[C@H]1CC5=C2C(=C(C=C5)O)O[C@H]3[C@H](C=C4)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: When pain is out of proportion.
[Johnson David E]Emerg Med Serv.2003 Sep;32(9):115-9. PMID: 14503162
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.