Search for drugs:
Typing the drug name to query
LAMOTRIGINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Acute Multiorgan Failure
- Multiorgan failure, which in some cases has been fatal or irreversible, has been observed in patients receiving lamotrigine. Fatalities associated with multiorgan failure and various degrees of hepatic failure have been reported in 2 of 3,796 adult patients and 4 of 2,435 pediatric patients who received lamotrigine in epilepsy clinical trials. No such fatalities have been reported in bipolar patients in clinical trials. Rare fatalities from multiorgan failure have also been reported in compassionate plea and postmarketing use. The majority of these deaths occurred in association with other serious medical events, including status epilepticus and overwhelming sepsis, and hantavirus, making it difficult to identify the initial cause.
- Additionally, 3 patients (a 45-year-old woman, a 3.5-year-old boy, and an 11-year-old girl) developed multiorgan dysfunction and disseminated intravascular coagulation 9 to 14 days after lamotrigine was added to their AED regimens. Rash and elevated transaminases were also present in all patients and rhabdomyolysis was noted in 2 patients. Both pediatric patients were receiving concomitant therapy with valproate, while the adult patient was being treated with carbamazepine and clonazepam. All patients subsequently recovered with supportive care after treatment with lamotrigine was discontinued.
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse events (not listed above in clinical trials or other sections of the prescribing information) have been identified during postapproval use of lamotrigine. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic: Agranulocytosis, hemolytic anemia.
- Gastrointestinal: Esophagitis.
- Hepatobiliary Tract and Pancreas: Pancreatitis.
- Immunologic:Lupus-like reaction, vasculitis.
- Lower Respiratory: Apnea.
- Musculoskeletal: Rhabdomyolysis has been observed in patients experiencing hypersensitivity reactions.
- Neurology: Exacerbation of Parkinsonian symptoms in patients with pre-existing Parkinson's disease, tics.
- Non-site Specific: Progressive immunosuppression.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
236
42676
Other ADRs
40072
14077207
Odds Ratio = 1.943
Drug Property Information
ATC Code(s):
- N03AX09 - lamotrigine
- N03AX - Other antiepileptics
- N03A - ANTIEPILEPTICS
- N03 - ANTIEPILEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:lamotrigine
Active Ingredient UNII:U3H27498KS
Drugbank ID:DB00555
PubChem Compound:3878
CAS Number:84057-84-1
Dosage Form(s):kit; tablet
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day N03AX09
Chemical Structure: 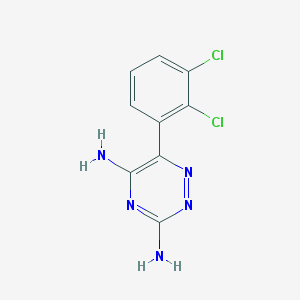

SMILE Code:
C1=CC(=C(C(=C1)Cl)Cl)C2=C(N=C(N=N2)N)N
C1=CC(=C(C(=C1)Cl)Cl)C2=C(N=C(N=N2)N)N
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Safety profile of lamotrigine in overdose.
[Alabi Akintunde,Todd Adam,Husband Andrew,Reilly Joe]Ther Adv Psychopharmacol.2016 Dec;6(6):369-381. doi: 10.1177/2045125316656707. Epub 2016 Aug 8. PMID: 28008350
2: Rhabdomyolysis after lamotrigine overdose: a case report and review of the literature.
[Karaoulanis Sokratis E,Syngelakis Markos,Fokas Konstantinos]Ann Gen Psychiatry.2016 Feb 24;15:6. doi: 10.1186/s12991-016-0093-3. eCollection 2016. PMID: 26913053
3: [Rhabdomyolysis in a Bipolar Adolescent. Analysis of Associated Factors].
[Restrepo Diana,Montoya Pablo,Giraldo Laura,Gaviria Génesis,Mejía Catalina]Rev Colomb Psiquiatr.2015 Jul-Sep;44(3):183-8. doi: 10.1016/j.rcp.2015.02.005. Epub 2015 Apr 23. PMID: 26578419
4: [Massive lamotrigine poisoning--case report].
[Kicka Mariusz,Kłopotowski Tomasz,Picheta Sebastian,Bazylewicz Anna,Miśkiewicz Łukasz]Przegl Lek.2011;68(8):543-5. PMID: 22010462
5: Life-threatening organ failure after lamotrigine therapy.
[Ferguson Lee P,Dargan Paul I,Hood Joanne L,Tibby Shane M]Pediatr Neurol.2009 May;40(5):392-4. doi: 10.1016/j.pediatrneurol.2008.11.018. PMID: 19380079
6: Atypical presentation of VLCAD deficiency associated with a novel ACADVL splicing mutation.
[Shchelochkov Oleg,Wong Lee-Jun,Shaibani Aziz,Shinawi Marwan]Muscle Nerve.2009 Mar;39(3):374-82. doi: 10.1002/mus.21157. PMID: 19208414
7: Seizures and altered mental status after lamotrigine overdose.
[Schwartz Michael D,Geller Robert J]Ther Drug Monit.2007 Dec;29(6):843-4. PMID: 18043485
8: Multiorgan dysfunction and disseminated intravascular coagulation in children receiving lamotrigine and valproic acid.
[Chattergoon D S,McGuigan M A,Koren G,Hwang P,Ito S]Neurology.1997 Nov;49(5):1442-4. PMID: 9371937
9: Seizures, ventricular tachycardia, and rhabdomyolysis as a result of ingestion of venlafaxine and lamotrigine.
[Peano C,Leikin J B,Hanashiro P K]Ann Emerg Med.1997 Nov;30(5):704-8. PMID: 9360588
10: Multisystem adverse reaction to lamotrigine.
[Schaub J E,Williamson P J,Barnes E W,Trewby P N]Lancet.1994 Aug 13;344(8920):481. PMID: 7914595
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.