Search for drugs:
Typing the drug name to query
SIMEPREVIR
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
- Established and Other Potentially Significant Drug Interactions
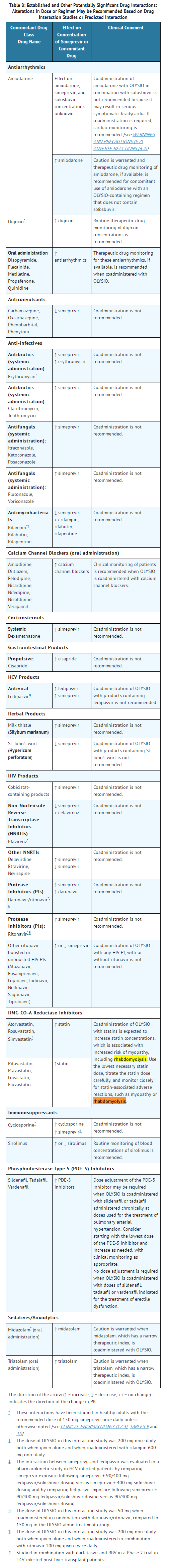
- Table 8 shows the established and other potentially significant drug interactions based on which alterations in dose or regimen of OLYSIO and/or coadministered drug may be recommended. Drugs that are not recommended for coadministration with OLYSIO are also included in Table 8.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
7
42905
Other ADRs
4033
14113246
Odds Ratio = 0.571
Drug Property Information
ATC Code(s):
- J05AE14 - simeprevir
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:simeprevir
Active Ingredient UNII:9WS5RD66HZ
Drugbank ID:DB06290
PubChem Compound:24873435
CAS Number:923604-59-5
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 150.0 mg/day J05AP05
Chemical Structure: 

SMILE Code:
CC1=C(C=CC2=C1N=C(C=C2O[C@@H]3C[C@@H]4[C@@H](C3)C(=O)N(CCCC/C=C\[C@@H]5C[C@]5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC
CC1=C(C=CC2=C1N=C(C=C2O[C@@H]3C[C@@H]4[C@@H](C3)C(=O)N(CCCC/C=C\[C@@H]5C[C@]5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Evaluation of the Pharmacokinetics and Renal Excretion of Simeprevir in Subjects with Renal Impairment.
[Ouwerkerk-Mahadevan Sivi,Beumont-Mauviel Maria,Mortier Steven,Peeters Monika,Verloes Rene,Truyers Carla,Mannens Geert,Wynant Inneke,Simion Alexandru]Drugs R D.2015 Sep;15(3):261-70. doi: 10.1007/s40268-015-0101-0. PMID: 26248593
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.