Search for drugs:
Typing the drug name to query
ASENAPINE MALEATE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Neuroleptic Malignant Syndrome
- A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs, including SAPHRIS. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
- The diagnostic evaluation of patients with this syndrome is complicated. It is important to exclude cases where the clinical presentation includes both serious medical illness (e.g. pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
- The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; 2) intensive symptomatic treatment and medical monitoring; and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS.
- If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
15
42897
Other ADRs
6500
14110779
Odds Ratio = 0.76
Drug Property Information
ATC Code(s):
- N05AH05 - asenapine maleate
- N05AH - "Diazepines, oxazepines and thiazepines"
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:asenapine maleate
Active Ingredient UNII:CU9463U2E2
Drugbank ID:DB06216
PubChem Compound:11954293
CAS Number:65576-45-6
Dosage Form(s):tablet
Route(s) Of Administrator:sublingual
Daily Dose:
- 20.0 mg/day N05AH05
Chemical Structure: 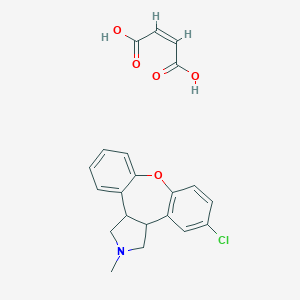

SMILE Code:
CN1CC2C(C1)C3=C(C=CC(=C3)Cl)OC4=CC=CC=C24.C(=C\C(=O)O)\C(=O)O
CN1CC2C(C1)C3=C(C=CC(=C3)Cl)OC4=CC=CC=C24.C(=C\C(=O)O)\C(=O)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.