Search for drugs:
Typing the drug name to query
MILNACIPRAN HYDROCHLORIDE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following additional adverse reactions have been identified from spontaneous reports of Savella received worldwide. These adverse reactions have been chosen for inclusion because of a combination of seriousness, frequency of reporting, or potential causal connection to Savella. However, because these adverse reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events include:
- Blood and Lymphatic System Disorders — leukopenia, neutropenia, thrombocytopenia
- Cardiac Disorders — supraventricular tachycardia, Takotsubo cardiomyopathy
- Eye Disorders — accommodation disorder
- Endocrine Disorders — hyperprolactinemia
- Gastrointestinal Disorders — acute pancreatitis
- Hepatobiliary Disorders — hepatitis
- Metabolism and Nutrition Disorders — anorexia, hyponatremia
- Musculoskeletal and Connective Tissue Disorders — rhabdomyolysis
- Nervous System Disorders — convulsions (including grand mal), loss of consciousness, Parkinsonism
- Psychiatric Disorders — aggression, anger, delirium, hallucination, homicidal ideation
- Renal and Urinary Disorders — acute renal failure
- Reproductive System and Breast Disorders — galactorrhea
- Skin Disorders — erythema multiforme, Stevens Johnson syndrome
- Vascular Disorders — hypertensive crisis
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
11
42901
Other ADRs
2639
14114640
Odds Ratio = 1.372
Drug Property Information
ATC Code(s):
- N06AX17 - milnacipran hydrochloride
- N06AX - Other antidepressants
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:milnacipran hydrochloride
Active Ingredient UNII:RNZ43O5WW5
Drugbank ID:DB04896
PubChem Compound:65833
CAS Number:92623-85-3
Dosage Form(s):kit; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 100.0 mg/day N06AX17
Chemical Structure: 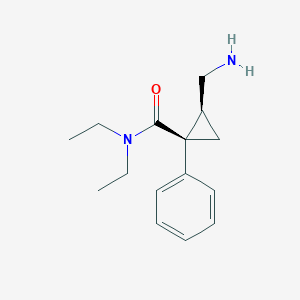

SMILE Code:
CCN(CC)C(=O)[C@@]1(C[C@@H]1CN)C2=CC=CC=C2
CCN(CC)C(=O)[C@@]1(C[C@@H]1CN)C2=CC=CC=C2
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.