Search for drugs:
Typing the drug name to query
METHAMPHETAMINE HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:2
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Manifestations of acute overdosage with methamphetamine include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmias, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning usually terminates in convulsions and coma.
- Consult with a Certified Poison Control Center regarding treatment for up to date guidance and advice. Management of acute methamphetamine intoxication is largely symptomatic and includes gastric evacuation, administration of activated charcoal, and sedation. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations in this regard.
- Acidification of urine increases methamphetamine excretion, but is believed to increase risk of acute renal failure if myoglobinuria is present. Intravenous phentolamine (Regitine) has been suggested for possible acute, severe hypertension, if this complicates methamphetamine overdosage. Usually a gradual drop in blood pressure will result when sufficient sedation has been achieved. Chlorpromazine has been reported to be useful in decreasing CNS stimulation and sympathomimetic effects.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
13
42899
Other ADRs
719
14116560
Odds Ratio = 5.95
Drug Property Information
ATC Code(s):
- N06BA03 - methamphetamine hydrochloride
- N06BA - Centrally acting sympathomimetics
- N06B - "PSYCHOSTIMULANTS, AGENTS USED FOR ADHD AND NOOTROPICS"
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:methamphetamine hydrochloride
Active Ingredient UNII:997F43Z9CV
Drugbank ID:DB01577
PubChem Compound:10836
CAS Number:537-46-2
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 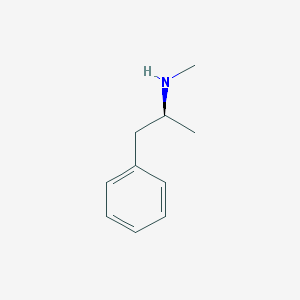

SMILE Code:
C[C@@H](CC1=CC=CC=C1)NC
C[C@@H](CC1=CC=CC=C1)NC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.