Search for drugs:
Typing the drug name to query
QUETIAPINE FUMARATE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- In clinical trials quetiapine was not associated with a persistent increase in QT intervals. However, the QT effect was not systematically evaluated in a thorough QT study. In post marketing experience there were cases reported of QT prolongation in patients who overdosed on quetiapine [see OVERDOSAGE (10.1)], in patients with concomitant illness, and in patients taking medicines known to cause electrolyte imbalance or increase QT interval.
- The use of quetiapine should be avoided in combination with other drugs that are known to prolong QTc including Class 1A antiarrythmics (e.g., quinidine, procainamide) or Class III antiarrythmics (e.g., amiodarone, sotalol), antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval (e.g., pentamidine, levomethadyl acetate, methadone).
- Quetiapine should also be avoided in circumstances that may increase the risk of occurrence of torsade de pointes and/or sudden death including (1) a history of cardiac arrhythmias such as bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
- Caution should also be exercised when quetiapine is prescribed in patients with increased risk of QT prolongation (e.g., cardiovascular disease, family history of QT prolongation, the elderly, congestive heart failure and heart hypertrophy).
- OVERDOSAGE
- Human Experience
- In clinical trials, survival has been reported in acute overdoses of up to 30 grams of quetiapine. Most patients who overdosed experienced no adverse reactions or recovered fully from the reported events. Death has been reported in a clinical trial following an overdose of 13.6 grams of quetiapine alone. In general, reported signs and symptoms were those resulting from an exaggeration of the drug’s known pharmacological effects, i.e., drowsiness, sedation, tachycardia, hypotension, and anticholinergic toxicity including coma and delirium. Patients with pre-existing severe cardiovascular disease may be at an increased risk of the effects of overdose [see WARNINGS AND PRECAUTIONS (5.12)]. One case, involving an estimated overdose of 9600 mg, was associated with hypokalemia and first degree heart block. In post-marketing experience, there were cases reported of QT prolongation with overdose.
- ADVERSE REACTIONS
- The following adverse reactions are discussed in more detail in other sections of the labeling:
- QT Prolongation [see WARNINGS AND PRECAUTIONS( 5.12)]
- CLINICAL PHARMACOLOGY
- Effect on QT Interval
- In clinical trials quetiapine was not associated with a persistent increase in QT intervals. However, the QT effect was not systematically evaluated in a thorough QT study. In post marketing experience there were cases reported of QT prolongation in patients who overdosed on quetiapine [see OVERDOSAGE (10.1)], in patients with concomitant illness, and in patients taking medicines known to cause electrolyte imbalance or increase QT interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
354
42558
Other ADRs
64317
14052962
Odds Ratio = 1.818
Drug Property Information
ATC Code(s):
- N05AH04 - quetiapine fumarate
- N05AH - "Diazepines, oxazepines and thiazepines"
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:quetiapine fumarate
Active Ingredient UNII:2S3PL1B6UJ
Drugbank ID:DB01224
PubChem Compound:5002
CAS Number:111974-69-7
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 400.0 mg/day N05AH04
Chemical Structure: 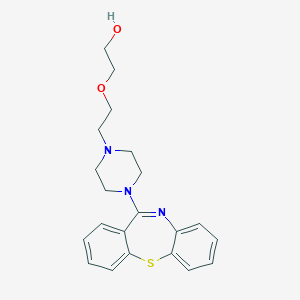

SMILE Code:
C1CN(CCN1CCOCCO)C2=NC3=CC=CC=C3SC4=CC=CC=C42
C1CN(CCN1CCOCCO)C2=NC3=CC=CC=C3SC4=CC=CC=C42
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [Rhabdomyolysis in a Bipolar Adolescent. Analysis of Associated Factors].
[Restrepo Diana,Montoya Pablo,Giraldo Laura,Gaviria Génesis,Mejía Catalina]Rev Colomb Psiquiatr.2015 Jul-Sep;44(3):183-8. doi: 10.1016/j.rcp.2015.02.005. Epub 2015 Apr 23. PMID: 26578419
2: N,N-Dimethyltryptamine-Induced Psychosis.
[Paterson Neil E,Darby W Connor,Sandhu Preetpal S]Clin Neuropharmacol.2015 Jul-Aug;38(4):141-3. doi: 10.1097/WNF.0000000000000078. PMID: 26166234
3: Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study.
[Hwang Y Joseph,Dixon Stephanie N,Reiss Jeffrey P,Wald Ron,Parikh Chirag R,Gandhi Sonja,Shariff Salimah Z,Pannu Neesh,Nash Danielle M,Rehman Faisal,Garg Amit X]Ann Intern Med.2014 Aug 19;161(4):242-8. doi: 10.7326/M13-2796. PMID: 25133360
4: Aseptic gingivitis related to quetiapine hemifumarate.
[Gahr M,Kölle M A,Freudenmann R W,Schönfeldt-Lecuona C]Pharmacopsychiatry.2013 Jan;46(1):39-40. doi: 10.1055/s-0032-1321906. Epub 2012 Aug 22. PMID: 22915485
5: Rhabdomyolysis secondary to quetiapine.
[Velasco-Montes Javier,Oriñuela-González Itziar,Sanjuán-López Ainhoa Z]Actas Esp Psiquiatr.2012 Mar-Apr;40(2):97-9. Epub 2012 Mar 1. PMID: 22508075
6: Neuroleptic malignant syndrome versus serotonin syndrome: the search for a diagnostic tool.
[Sokoro Abdulrazaq A H,Zivot Joel,Ariano Robert E]Ann Pharmacother.2011 Sep;45(9):e50. doi: 10.1345/aph.1P787. Epub 2011 Aug 30. PMID: 21878660
7: Comment on: low-dose quetiapine-induced severe rhabdomyolysis.
[Ceri Mevlut,Unverdi Selman,Altay Mustafa,Duranay Murat]Ren Fail.2011;33(4):463-4. doi: 10.3109/0886022X.2011.568141. PMID: 21529278
8: An uncommonly recognized cause of rhabdomyolysis after quetiapine intoxication.
[Dickmann Julian Robert Mario,Dickmann Laura Maria]Am J Emerg Med.2010 Nov;28(9):1060.e1-2. doi: 10.1016/j.ajem.2010.01.025. Epub 2010 May 4. PMID: 20825857
9: Rhabdomyolysis after low-dose quetiapine in a patient with Parkinson's disease with drug-induced psychosis: a case report.
[Stephani Caspar,Trenkwalder Claudia]Mov Disord.2010 Apr 30;25(6):790-1. doi: 10.1002/mds.23015. PMID: 20198646
10: Quetiapine and elevated creatine phosphokinase (CK).
[Plesnicar B K,Lasic J K,Plesnicar A]Pharmacopsychiatry.2007 Sep;40(5):203-4. PMID: 17874354
11: Possible case of quetiapine-induced rhabdomyolysis in a patient with depression treated with fluoxetine.
[Himmerich Hubertus,Ehrlinger Martin,Hackenberg Melanie,Löhr Birgit,Nickel Thomas]J Clin Psychopharmacol.2006 Dec;26(6):676-7. PMID: 17110835
12: A young onset Parkinson's patient: a case study.
[Jung Sharon K]J Neurosci Nurs.2004 Oct;36(5):273-7. PMID: 15524245
13: Quetiapine overdose and severe rhabdomyolysis.
[Smith Richard P,Puckett Ben N,Crawford Jennifer,Elliott Richard L]J Clin Psychopharmacol.2004 Jun;24(3):343. PMID: 15118490
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.