Search for drugs:
Typing the drug name to query
RITONAVIR
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
- Established and Other Potentially Significant Drug Interactions
- figure
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once-daily) controlled crossover study in 45 healthy adults, with 10 measurements over 12 hours on Day 3. The maximum mean (95% upper confidence bound) time-matched difference in QTcF from placebo after baseline correction was 5.5 (7.6) milliseconds (msec) for 400 mg twice-daily ritonavir. Ritonavir 400 mg twice daily resulted in Day 3 ritonavir exposure that was approximately 1.5 fold higher than observed with ritonavir 600 mg twice-daily dose at steady state.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
42
42870
Other ADRs
8568
14108711
Odds Ratio = 1.614
Drug Property Information
ATC Code(s):
- J05AX67 - ritonavir
- J05AX - Other antivirals
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR10 - ritonavir
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AE03 - ritonavir
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AX66 - ritonavir
- J05AX - Other antivirals
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:ritonavir
Active Ingredient UNII:O3J8G9O825
Drugbank ID:DB00503
PubChem Compound:392622
CAS Number:155213-67-5
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 1200.0 mg/day J05AE03
Chemical Structure: 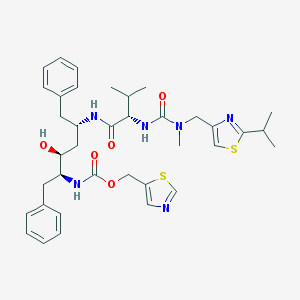

SMILE Code:
CC(C)C1=NC(=CS1)CN(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC2=CC=CC=C2)C[C@@H]([C@H](CC3=CC=CC=C3)NC(=O)OCC4=CN=CS4)O
CC(C)C1=NC(=CS1)CN(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC2=CC=CC=C2)C[C@@H]([C@H](CC3=CC=CC=C3)NC(=O)OCC4=CN=CS4)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [Rhabdomyolysis and severe hepatotoxicity due to a drug-drug interaction between ritonavir and simvastatin. Could we use the most cost-effective statin in all human immunodeficiency virus-infected patients?].
[Bastida Carla,Also Maria Antonia,Pericas Juan Manuel,Letang Emili,Tuset Montse,Miró Josep Maria]Enferm Infecc Microbiol Clin.2014 Nov;32(9):579-82. doi: 10.1016/j.eimc.2014.03.014. Epub 2014 Jun 7. PMID: 24913991
2: Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors.
[Chauvin Benoit,Drouot Sylvain,Barrail-Tran Aurélie,Taburet Anne-Marie]Clin Pharmacokinet.2013 Oct;52(10):815-31. doi: 10.1007/s40262-013-0075-4. PMID: 23703578
3: Rhabdomyolysis in an HIV-infected patient with impaired renal function concomitantly treated with rosuvastatin and lopinavir/ritonavir.
[de Kanter Clara T M M,Keuter Monique,van der Lee Manon J,Koopmans Peter P,Burger David M]Antivir Ther.2011;16(3):435-7. doi: 10.3851/IMP1747. PMID: 21555828
4: Severe rhabdomyolysis due to rosuvastatin in a liver transplant subject with human immunodeficiency virus and immunosuppressive therapy-related dyslipidemia.
[Moreno Ana,Fortún Jesús,Graus Javier,Rodriguez-Gandía Miguel A,Quereda Carmen,Pérez-Elías María J,Nuño Javier,Wikman Philip,Moreno Santiago,Bárcena Rafael]Liver Transpl.2011 Mar;17(3):331-3. doi: 10.1002/lt.22225. PMID: 21384516
5: Tolerability of HIV postexposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir tablet formulation.
[Tosini William,Muller Philippe,Prazuck Thierry,Benabdelmoumen Ghania,Peyrouse Eric,Christian Bernard,Quertainmont Yann,Bouvet Elisabeth,Rabaud Christian]AIDS.2010 Sep 24;24(15):2375-80. doi: 10.1097/QAD.0b013e32833dfad1. PMID: 20729709
6: Short term treatment with clarithromycin resulting in colchicine-induced rhabdomyolysis.
[McKinnell James,Tayek John A]J Clin Rheumatol.2009 Sep;15(6):303-5. doi: 10.1097/RHU.0b013e3181bbbcd7. PMID: 19734738
7: Rhabdomyolysis in an HIV-infected patient on anti-retroviral therapy precipitated by high-dose pravastatin.
[Mikhail Nasser,Iskander Elizabeth,Cope Dennis]Curr Drug Saf.2009 May;4(2):121-2. PMID: 19442105
8: Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance.
[Neuvonen Pertti J,Niemi Mikko,Backman Janne T]Clin Pharmacol Ther.2006 Dec;80(6):565-81. PMID: 17178259
9: Clinical pharmacokinetics of atorvastatin.
[Lennernäs Hans]Clin Pharmacokinet.2003;42(13):1141-60. PMID: 14531725
10: Drug-induced rhabdomyolysis after concomitant use of clarithromycin, atorvastatin, and lopinavir/ritonavir in a patient with HIV.
[Mah Ming Jinell B,Gill M John]AIDS Patient Care STDS.2003 May;17(5):207-10. PMID: 12816614
11: Rhabdomyolysis due to probable interaction between simvastatin and ritonavir.
[Cheng Cindy H,Miller Christine,Lowe Christopher,Pearson Vincent Earl]Am J Health Syst Pharm.2002 Apr 15;59(8):728-30. PMID: 11977859
12: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.
[Dresser G K,Spence J D,Bailey D G]Clin Pharmacokinet.2000 Jan;38(1):41-57. PMID: 10668858
13: Two episodes of acute renal failure, rhabdomyolysis, and severe hepatitis in an AIDS patient successively treated with ritonavir and indinavir.
[Benveniste O,Longuet P,Duval X,Le Moing V,Leport C,Vildé J L]Clin Infect Dis.1999 May;28(5):1180-1. PMID: 10452668
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.