Search for drugs:
Typing the drug name to query
OLANZAPINE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Clinical Trials Experience
- ECG Changes In pooled studies of adults as well as pooled studies of adolescents, there were no significant differences between olanzapine and placebo in the proportions of patients experiencing potentially important changes in ECG parameters, including QT, QTc (Fridericia corrected), and PR intervals. Olanzapine use was associated with a mean increase in heart rate compared to placebo (adults: +2.4 beats per minute vs no change with placebo; adolescents: +6.3 beats per minute vs -5.1 beats per minute with placebo). This increase in heart rate may be related to olanzapine potential for inducing orthostatic changes [see Warnings and Precautions ( 5.7)] .
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
618
42294
Other ADRs
39962
14077317
Odds Ratio = 5.148
Drug Property Information
ATC Code(s):
- N05AH03 - olanzapine
- N05AH - "Diazepines, oxazepines and thiazepines"
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:olanzapine
Active Ingredient UNII:N7U69T4SZR
Drugbank ID:DB00334
PubChem Compound:4585
CAS Number:132539-06-1
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 10.0 mg/day N05AH03
Chemical Structure: 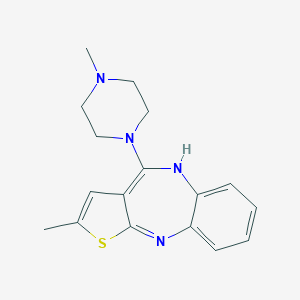

SMILE Code:
CC1=CC2=C(NC3=CC=CC=C3N=C2S1)N4CCN(CC4)C
CC1=CC2=C(NC3=CC=CC=C3N=C2S1)N4CCN(CC4)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Neuroleptic malignant syndrome following catatonia: Vigilance is the price of antipsychotic prescription.
[Reilly Thomas J,Cross Sean,Taylor David M,Haslam Richard,Tomlin Sophie C,Gaastra Benjamin]SAGE Open Med Case Rep.2017 Mar 31;5:2050313X17695999. doi: 10.1177/2050313X17695999. eCollection 2017. PMID: 28491312
2: A Retrospective Cohort Study of Acute Kidney Injury Risk Associated with Antipsychotics.
[Jiang Yawen,McCombs Jeffrey S,Park Susie H]CNS Drugs.2017 Apr;31(4):319-326. doi: 10.1007/s40263-017-0421-4. PMID: 28290080
3: Serotonin Syndrome Induced by Combined Use of Mirtazapine and Olanzapine Complicated with Rhabdomyolysis, Acute Renal Failure, and Acute Pulmonary Edema-A Case Report.
[Wu Chi-Shun,Tong Show-Hwa,Ong Cheung-Ter,Sung Sheng-Feng]Acta Neurol Taiwan.2015 Dec;24(4):117-21. PMID: 27333965
4: Delayed-onset rhabdomyolysis related to olanzapine: a case report.
[Lee Yen-Feng,Mao Wei-Chung,Tai Yueh-Ming,Chang Hsin-An,Kao Yu-Chen,Huang San-Yuan,Tzeng Nian-Sheng]Singapore Med J.2016 May;57(5):279. doi: 10.11622/smedj.2016094. PMID: 27212263
5: Rhabdomyolysis after lamotrigine overdose: a case report and review of the literature.
[Karaoulanis Sokratis E,Syngelakis Markos,Fokas Konstantinos]Ann Gen Psychiatry.2016 Feb 24;15:6. doi: 10.1186/s12991-016-0093-3. eCollection 2016. PMID: 26913053
6: Electroconvulsive in a Schizophrenic Patient With Neuroleptic Malignant Syndrome and Rhabdomyolysis.
[San Gabriel Maria Chona P,Eddula-Changala Bharathi,Tan Yonghong,Longshore Carrol T]J ECT.2015 Sep;31(3):197-200. doi: 10.1097/YCT.0000000000000184. PMID: 25243752
7: Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study.
[Hwang Y Joseph,Dixon Stephanie N,Reiss Jeffrey P,Wald Ron,Parikh Chirag R,Gandhi Sonja,Shariff Salimah Z,Pannu Neesh,Nash Danielle M,Rehman Faisal,Garg Amit X]Ann Intern Med.2014 Aug 19;161(4):242-8. doi: 10.7326/M13-2796. PMID: 25133360
8: Olanzapine-induced diabetic ketoacidosis and neuroleptic malignant syndrome with rhabdomyolysis: a case report.
[Sa Young Kyoung,Yang Hyeon,Jung Hee Kyoung,Son Jang Won,Lee Seong Su,Kim Seong Rae,Cha Bong Yeon,Son Ho Young,Pae Chi-Un,Yoo Soon Jib]Endocrinol Metab (Seoul).2013 Mar;28(1):70-5. doi: 10.3803/EnM.2013.28.1.70. Epub 2013 Mar 25. PMID: 24396655
9: Rhabdomyolysis reported for children and adolescents treated with antipsychotic medicines: a case series analysis.
[Star Kristina,Iessa Noha,Almandil Noor B,Wilton Lynda,Curran Sarah,Edwards I Ralph,Wong Ian C K]J Child Adolesc Psychopharmacol.2012 Dec;22(6):440-51. doi: 10.1089/cap.2011.0134. PMID: 23234587
10: [Intensive care treatment for neuroleptic malignant syndrome].
[Hensel Mario,Böhler Klaus,Marnitz Rudolf,Binder Christian,von Brevern Michael]Anasthesiol Intensivmed Notfallmed Schmerzther.2010 Jul;45(7-8):448-55. doi: 10.1055/s-0030-1262471. Epub 2010 Jul 21. PMID: 20665353
11: Acute camptocormia induced by olanzapine: a case report.
[Robert Florence,Koenig Martial,Robert Aurélie,Boyer Stéphane,Cathébras Pascal,Camdessanché Jean-Philippe]J Med Case Rep.2010 Jun 25;4:192. doi: 10.1186/1752-1947-4-192. PMID: 20579377
12: [Patient with encephalitis presenting with olanzapine-responsive malignant catatonia].
[Suzuki Hayato,Fukushima Takao,Makino Kunihiko,Kuwabara Takeo]Rinsho Shinkeigaku.2010 May;50(5):329-31. PMID: 20535983
13: Rhabdomyolysis associated with olanzapine treatment in a child with Autism.
[Karakaya Pakize,Yiş Uluç,Kurul Semra Hz,Türkmen Mehmet Atilla]Pediatr Emerg Care.2010 Jan;26(1):41-2. doi: 10.1097/PEC.0b013e3181c39a22. PMID: 20065830
14: Hypothermia and rhabdomyolysis following olanzapine injection in an adolescent with schizophreniform disorder.
[Hung Chi-Fa,Huang Tsan-Yu,Lin Pao-Yen]Gen Hosp Psychiatry.2009 Jul-Aug;31(4):376-8. doi: 10.1016/j.genhosppsych.2008.09.009. Epub 2008 Oct 18. PMID: 19555799
15: [Olanzapine induced rhabdomyolysis and serum creatine kinase increase].
[Ribeyron S,Guy C,Koenig M,Cathébras P]Rev Med Interne.2009 Jun;30(6):477-85. doi: 10.1016/j.revmed.2008.12.024. PMID: 19307047
16: Hyperthermia and rhabdomyolysis in an adolescent treated with topiramate and olanzapine.
[Strawn Jeffrey R,Adler Caleb M,Strakowski Stephen M,DelBello Melissa P]J Child Adolesc Psychopharmacol.2008 Feb;18(1):116-8. doi: 10.1089/cap.2007.0101. PMID: 18294095
17: Fatal toxicity of drugs used in psychiatry.
[Flanagan Robert J]Hum Psychopharmacol.2008 Jan;23 Suppl 1:43-51. PMID: 18098225
18: Olanzapine overdose is associated with acute muscle toxicity.
[Waring W S,Wrate J,Bateman D N]Hum Exp Toxicol.2006 Dec;25(12):735-40. PMID: 17286152
19: Cerebral salt-wasting syndrome in a patient with neuroleptic malignant syndrome.
[Lenhard Thorsten,Külkens Sonja,Schwab Stefan]Arch Neurol.2007 Jan;64(1):122-5. PMID: 17210819
20: Successful switch to olanzapine after rhabdomyolysis caused by water intoxication and clozapine use.
[Tényi T,Vörös V]Pharmacopsychiatry.2006 Jul;39(4):157-8. PMID: 16871473
21: Adverse drug reactions in Canada.
Can Fam Physician.2005 May;51:710-2. PMID: 15934277
22: Olanzapine-induced acute rhabdomyolysis-a case report.
[Baumgart U,Schmid R,Spiessl H]Pharmacopsychiatry.2005 Jan;38(1):36-7. PMID: 15706465
23: Neuroleptic malignant syndrome in an adolescent after brief exposure to olanzapine.
[Hanft Alan,Eggleston Christopher F,Bourgeois James A]J Child Adolesc Psychopharmacol.2004 Fall;14(3):481-7. PMID: 15650507
24: [Rhabdomyolysis associated with respiratory infection in chronic psychiatric patients during neuroleptic treatment].
[Pezza M,Busiello L,Palmese S,Cascella M,Di Domenico M G,De Robertis E]Minerva Anestesiol.2003 Jun;69(6):591-6. PMID: 14564256
25: Olanzapine-induced rhabdomyolysis.
[Rosebraugh C J,Flockhart D A,Yasuda S U,Woosley R L]Ann Pharmacother.2001 Sep;35(9):1020-3. PMID: 11573848
26: Olanzapine and rhabdomyolysis.
[Shuster J]Nursing.2000 Sep;30(9):87. PMID: 11022553
27: Fatal status epilepticus associated with olanzapine therapy.
[Wyderski R J,Starrett W G,Abou-Saif A]Ann Pharmacother.1999 Jul-Aug;33(7-8):787-9. PMID: 10466904
28: Marked elevation of serum creatine kinase associated with olanzapine therapy.
[Marcus E L,Vass A,Zislin J]Ann Pharmacother.1999 Jun;33(6):697-700. PMID: 10410183
29: Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment.
[Meltzer H Y,Cola P A,Parsa M]Neuropsychopharmacology.1996 Oct;15(4):395-405. PMID: 8887994
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.