Search for drugs:
Typing the drug name to query
IRBESARTAN
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Post-Marketing Experience
- The following adverse reactions have been identified during post-approval use of Irbesartan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or to establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to Irbesartan.
- The following have been reported: urticaria; angioedema (involving swelling of the face, lips, pharynx, and/or tongue); increased liver function tests; jaundice; hepatitis; hyperkalemia, and thrombocytopenia.
- Impaired renal function, including cases of renal failure, has been reported.
- Cases of increased CPK and rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
59
42853
Other ADRs
6062
14111217
Odds Ratio = 3.205
Drug Property Information
ATC Code(s):
- C09DB05 - irbesartan
- C09DB - Angiotensin II antagonists and calcium channel blockers
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09DA04 - irbesartan
- C09DA - Angiotensin II antagonists and diuretics
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09CA04 - irbesartan
- C09CA - "Angiotensin II antagonists, plain"
- C09C - "ANGIOTENSIN II ANTAGONISTS, PLAIN"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:irbesartan
Active Ingredient UNII:J0E2756Z7N
Drugbank ID:DB01029
PubChem Compound:3749
CAS Number:138402-11-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 150.0 mg/day C09CA04
Chemical Structure: 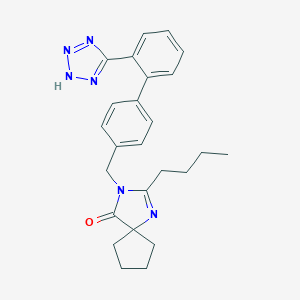

SMILE Code:
CCCCC1=NC2(CCCC2)C(=O)N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5
CCCCC1=NC2(CCCC2)C(=O)N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rhabdomyolysis associated with phentermine.
[Steidl Kelly E,Darko William,Probst Luke A,Noviasky John A,Nasser Samer]Am J Health Syst Pharm.2010 Nov 15;67(22):1929-32. doi: 10.2146/ajhp090395. PMID: 21048209
2: Hypokalemia induced myopathy as first manifestation of primary hyperaldosteronism - an elderly patient with unilateral adrenal hyperplasia: a case report.
[Kotsaftis Panagiotis,Savopoulos Christos,Agapakis Dimitrios,Ntaios George,Tzioufa Valentini,Papadopoulos Vasilios,Fahantidis Epaminondas,Hatzitolios Apostolos]Cases J.2009 Jul 16;2:6813. doi: 10.4076/1757-1626-2-6813. PMID: 19829865
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.