Search for drugs:
Typing the drug name to query
DAPTOMYCIN
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Myopathy and Rhabdomyolysis
- Myopathy, defined as muscle aching or muscle weakness in conjunction with increases in creatine phosphokinase (CPK) values to greater than 10 times the upper limit of normal (ULN), has been reported with the use of Daptomycin for injection. Rhabdomyolysis, with or without acute renal failure, has been reported [see ADVERSE REACTIONS (6.2)].
- Patients receiving Daptomycin for injection should be monitored for the development of muscle pain or weakness, particularly of the distal extremities. In patients who receive Daptomycin for injection, CPK levels should be monitored weekly, and more frequently in patients who received recent prior or concomitant therapy with an HMG-CoA reductase inhibitor or in whom elevations in CPK occur during treatment with Daptomycin for injection.
- In patients with renal impairment, both renal function and CPK should be monitored more frequently than once weekly [see USE IN SPECIFIC POPULATIONS (8.6) and CLINICAL PHARMACOLOGY (12.3)].
- In Phase 1 studies and Phase 2 clinical trials, CPK elevations appeared to be more frequent when Daptomycin for injection was dosed more than once daily. Therefore, Daptomycin for injection should not be dosed more frequently than once a day.
- Daptomycin for injection should be discontinued in patients with unexplained signs and symptoms of myopathy in conjunction with CPK elevations to levels >1,000 U/L (~5× ULN), and in patients without reported symptoms who have marked elevations in CPK, with levels >2,000 U/L (≥10× ULN). In addition, consideration should be given to suspending agents associated with rhabdomyolysis, such as HMG-CoA reductase inhibitors, temporarily in patients receiving Daptomycin for injection [see DRUG INTERACTIONS (7.1)].
- ADVERSE REACTIONS
- The following adverse reactions are described, or described in greater detail, in other sections:
- - Anaphylaxis/hypersensitivity reactions [see WARNINGS AND PRECAUTIONS (5.1)]
- - Myopathy and rhabdomyolysis [see WARNINGS AND PRECAUTIONS (5.2)]
- - Eosinophilic pneumonia [see WARNINGS AND PRECAUTIONS (5.3)]
- - Peripheral neuropathy [see WARNINGS AND PRECAUTIONS (5.4)]
- - Increased International Normalized Ratio (INR)/prolonged prothrombin time [see WARNINGS AND PRECAUTIONS (5.9) and DRUG INTERACTIONS (7.2)]
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Post-Marketing Experience
- The following adverse reactions have been identified during postapproval use of Daptomycin for injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and lymphatic system disorders: anemia
- General and administration site conditions: pyrexia
- Immune System Disorders: anaphylaxis; hypersensitivity reactions, including angioedema, drug rash with eosinophilia and systemic symptoms (DRESS), pruritus, hives, shortness of breath, difficulty swallowing, truncal erythema, and pulmonary eosinophilia [see CONTRAINDICATIONS (4), WARNINGS AND PRECAUTIONS (5.1)]
- Infections and Infestations: Clostridium difficile–associated diarrhea [see WARNINGS AND PRECAUTIONS (5.6)]
- Musculoskeletal Disorders: myoglobin increased; rhabdomyolysis (some reports involved patients treated concurrently with Daptomycin for injection and HMG-CoA reductase inhibitors) [see WARNINGS AND PRECAUTIONS (5.2), DRUG INTERACTIONS (7.1), and CLINICAL PHARMACOLOGY (12.3)]
- Respiratory, Thoracic, and Mediastinal Disorders: cough, eosinophilic pneumonia [see WARNINGS AND PRECAUTIONS (5.3)]
- Nervous System Disorders: peripheral neuropathy [see WARNINGS AND PRECAUTIONS (5.4)]
- Skin and Subcutaneous Tissue Disorders: serious skin reactions, including Stevens-Johnson syndrome and vesiculobullous rash (with or without mucous membrane involvement), acute generalized exanthematous pustulosis
- Gastrointestinal Disorders: nausea, vomiting
- Renal and urinary disorders: acute kidney injury, renal insufficiency, and renal failure
- Special Senses: visual disturbances
- NONCLINICAL TOXICOLOGY
- Animal Toxicology and/or Pharmacology
- Adult Animals
- In animals, daptomycin administration has been associated with effects on skeletal muscle. However, there were no changes in cardiac or smooth muscle. Skeletal muscle effects were characterized by microscopic degenerative/regenerative changes and variable elevations in creatine phosphokinase (CPK). No fibrosis or rhabdomyolysis was evident in repeat-dose studies up to the highest doses tested in rats (150 mg/kg/day) and dogs (100 mg/kg/day). The degree of skeletal myopathy showed no increase when treatment was extended from 1 month to up to 6 months. Severity was dose-dependent. All muscle effects, including microscopic changes, were fully reversible within 30 days following the cessation of dosing.
- In adult animals, effects on peripheral nerve (characterized by axonal degeneration and frequently accompanied by significant losses of patellar reflex, gag reflex, and pain perception) were observed at daptomycin doses higher than those associated with skeletal myopathy. Deficits in the dogs’ patellar reflexes were seen within 2 weeks after the start of treatment at 40 mg/kg/day (9 times the human Cmax at the 6 mg/kg/day dose), with some clinical improvement noted within 2 weeks after the cessation of dosing. However, at 75 mg/kg/day for 1 month, 7 of 8 dogs failed to regain full patellar reflex responses within a 3-month recovery period. In a separate study in dogs receiving doses of 75 and 100 mg/kg/day for 2 weeks, minimal residual histological changes were noted at 6 months after the cessation of dosing. However, recovery of peripheral nerve function was evident.
- Tissue distribution studies in rats showed that daptomycin is retained in the kidney but appears to penetrate the blood-brain barrier only minimally following single and multiple doses.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
264
42648
Other ADRs
5058
14112221
Odds Ratio = 17.272
Drug Property Information
ATC Code(s):
- J01XX09 - daptomycin
- J01XX - Other antibacterials
- J01X - OTHER ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:daptomycin
Active Ingredient UNII:NWQ5N31VKK
Drugbank ID:DB00080
PubChem Compound:16134395
CAS Number:103060-53-3
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 280.0 mg/day J01XX09
Chemical Structure: 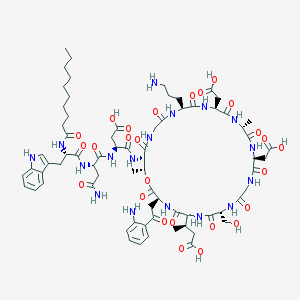

SMILE Code:
CCCCCCCCCC(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)N[C@H]3[C@H](OC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC3=O)CCCN)CC(=O)O)C)CC(=O)O)CO)[C@H](C)CC(=O)O)CC(=O)C4=CC=CC=C4N)C
CCCCCCCCCC(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)N[C@H]3[C@H](OC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC3=O)CCCN)CC(=O)O)C)CC(=O)O)CO)[C@H](C)CC(=O)O)CC(=O)C4=CC=CC=C4N)C
Reference
COHORT STUDY:
1: Safety and effectiveness of daptomycin across a hospitalized obese population: results of a multicenter investigation in the southeastern United States.
[Bookstaver PB, Bland CM, Qureshi ZP, Faulkner-Fennell CM, Sheldon MA, Caulder CR, Hartis C; SERGE-45 Investigators,Pharmacotherapy. 2013 Dec;33(12):1322-30.]ABSTRACT
STUDY OBJECTIVE: Data are limited for antimicrobial outcomes in obese patients. This study investigated the safety and clinical outcomes of daptomycin therapy in a hospitalized obese population in the southeastern United States.
DESIGN: Multicenter retrospective cohort study.
SETTING: Thirteen hospitals in the southeastern United States.
PATIENTS: A total of 126 hospitalized adult obese patients (body mass index [BMI] more than 30 kg/m(2) ) admitted from January 2005 through May 2010 who received daptomycin dosed on actual body weight for any indication for a minimum of 7 days.
MEASUREMENTS AND MAIN RESULTS: Primary safety outcomes included incidence of creatine phosphokinase (CPK) elevations more than 1000 units/L, more than 500 units/L, myalgias, and discontinuation of therapy due to adverse drug events (ADEs). Patients were stratified by BMI class (I, II, or III) for analyses. The average weight was 121 kg, and 39% of patients were considered morbidly obese. Factors associated with an increased risk of primary safety outcomes were assessed through regression analysis. Clinical effectiveness was evaluated as a secondary outcome. CPK elevations more than 1000 units/L occurred in 8.4% of evaluable patients and specifically in 1 (3.6%), 3 (10.3%), and 4 (10.5%) patients in BMI class I, II, and III, respectively (p=0.554). CPK elevations more than 500 units/L occurred in 13.7% of patients with no statistically significant difference noted across BMI classes. Discontinuation due to ADEs occurred in 8 patients (6.3%). One patient developed rhabdomyolysis on day 9 of therapy. Clinical effectiveness was documented in 71% of patients and was consistent across BMI classes.
CONCLUSION: Although elevations in CPK increased in high-risk obese patients on daptomycin, discontinuation rates due to ADEs remained low. Further evaluation in a prospective trial is warranted.
PMID: 23712701
DESIGN: Multicenter retrospective cohort study.
SETTING: Thirteen hospitals in the southeastern United States.
PATIENTS: A total of 126 hospitalized adult obese patients (body mass index [BMI] more than 30 kg/m(2) ) admitted from January 2005 through May 2010 who received daptomycin dosed on actual body weight for any indication for a minimum of 7 days.
MEASUREMENTS AND MAIN RESULTS: Primary safety outcomes included incidence of creatine phosphokinase (CPK) elevations more than 1000 units/L, more than 500 units/L, myalgias, and discontinuation of therapy due to adverse drug events (ADEs). Patients were stratified by BMI class (I, II, or III) for analyses. The average weight was 121 kg, and 39% of patients were considered morbidly obese. Factors associated with an increased risk of primary safety outcomes were assessed through regression analysis. Clinical effectiveness was evaluated as a secondary outcome. CPK elevations more than 1000 units/L occurred in 8.4% of evaluable patients and specifically in 1 (3.6%), 3 (10.3%), and 4 (10.5%) patients in BMI class I, II, and III, respectively (p=0.554). CPK elevations more than 500 units/L occurred in 13.7% of patients with no statistically significant difference noted across BMI classes. Discontinuation due to ADEs occurred in 8 patients (6.3%). One patient developed rhabdomyolysis on day 9 of therapy. Clinical effectiveness was documented in 71% of patients and was consistent across BMI classes.
CONCLUSION: Although elevations in CPK increased in high-risk obese patients on daptomycin, discontinuation rates due to ADEs remained low. Further evaluation in a prospective trial is warranted.
OTHER REFERENCE(S):
1: Daptomycin treatment in patients with resistant staphylococcal periprosthetic joint infection.
[Chang Yu-Jui,Lee Mel S,Lee Chen-Hsiang,Lin Po-Chun,Kuo Feng-Chih]BMC Infect Dis.2017 Nov 29;17(1):736. doi: 10.1186/s12879-017-2842-6. PMID: 29187163
2: Methicillin-resistant Staphylococcal periprosthetic joint infections can be effectively controlled by systemic and local daptomycin.
[Kuo Feng-Chih,Yen Shih-Hsiang,Peng Kuo-Ti,Wang Jun-Wen,Lee Mel S]BMC Infect Dis.2016 Feb 1;16:48. doi: 10.1186/s12879-016-1366-9. PMID: 26830838
3: Possible Hepatotoxicity Associated With Daptomycin: A Case Report and Literature Review.
[Mo Yoonsun,Nehring Fletcher,Jung Andrew H,Housman Seth T]J Pharm Pract.2016 Jun;29(3):253-6. doi: 10.1177/0897190015625403. Epub 2016 Jan 13. PMID: 26763341
4: Daptomycin-induced rhabdomyolysis and acute liver injury.
[King S Travis,Walker Erica D,Cannon Colleen G,Finley Richard W]Scand J Infect Dis.2014 Jul;46(7):537-40. doi: 10.3109/00365548.2014.901555. Epub 2014 May 7. PMID: 24801642
5: Normalization of creatine kinase values in a case of rhabdomyolysis during daptomycin treatment.
[Ferrández Olivia,Urbina Olatz,Luque Sònia,Espona Mercè,Berenguer Nuria,Grau Santiago]Indian J Pharmacol.2013 Mar-Apr;45(2):193-4. doi: 10.4103/0253-7613.108321. PMID: 23716901
6: Safety and effectiveness of daptomycin across a hospitalized obese population: results of a multicenter investigation in the southeastern United States.
[Bookstaver P Brandon,Bland Christopher M,Qureshi Zaina P,Faulkner-Fennell Carmen M,Sheldon Margrit A,Caulder Celeste R,Hartis Charles,SERGE-45 Investigators]Pharmacotherapy.2013 Dec;33(12):1322-30. doi: 10.1002/phar.1298. Epub 2013 May 26. PMID: 23712701
7: Statins and daptomycin: safety assessment of concurrent use and evaluation of drug interaction liability.
[Golightly Larry K,Barber Gerard R,Barron Michelle A,Page Robert L]Drug Metabol Drug Interact.2013;28(1):49-58. doi: 10.1515/dmdi-2012-0033. PMID: 23314530
8: Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections.
[Corona Pérez-Cardona Pablo S,Barro Ojeda Victor,Rodriguez Pardo Dolors,Pigrau Serrallach Carlos,Guerra Farfán Ernesto,Amat Mateu Carles,Flores Sanchez Xavier]J Antimicrob Chemother.2012 Jul;67(7):1749-54. doi: 10.1093/jac/dks119. Epub 2012 Apr 17. PMID: 22511636
9: [Severe daptomycin-induced myopathy].
[Ferrera Carlos,Vilacosta Isidre,Vivas David,Olmos Carmen]Med Clin (Barc).2012 Jun 30;139(3):138-9. doi: 10.1016/j.medcli.2011.12.001. Epub 2012 Jan 27. PMID: 22285497
10: Safety analysis of high dose (>6 mg/kg/day) daptomycin in patients with concomitant statin therapy.
[Parra-Ruiz J,Dueñas-Gutiérrez C,Tomás-Jiménez C,Linares-Palomino J P,Garrido-Gomez J,Hernández-Quero J]Eur J Clin Microbiol Infect Dis.2012 Aug;31(8):1771-4. doi: 10.1007/s10096-011-1500-y. Epub 2011 Dec 8. PMID: 22160888
11: Rhabdomyolysis associated with the co-administration of daptomycin and pegylated interferon α-2b and ribavirin in a patient with hepatitis C.
[Colomba Claudia,Rubino Raffaella,Siracusa Lucia,Mazzola Giovanni,Titone Lucina]J Antimicrob Chemother.2012 Jan;67(1):249-50. doi: 10.1093/jac/dkr398. Epub 2011 Sep 29. PMID: 21965434
12: Successful re-challenge of daptomycin therapy after initial rhabdomyolysis with co-administration of simvastatin.
[Bland Christopher M,Bookstaver P Brandon,Thomas Sanil]Int J Antimicrob Agents.2011 Dec;38(6):549-50. doi: 10.1016/j.ijantimicag.2011.08.003. Epub 2011 Sep 29. PMID: 21958456
13: Administration interval and daptomycin toxicity: a case report of rhabdomyolysis.
[Sbrana F,Di Paolo A,Pasanisi E M,Tagliaferri E,Arvia C,Puntoni M,Leonildi A,Bigazzi F,Danesi R,Rovai D,Tascini C,Menichetti F]J Chemother.2010 Dec;22(6):434-5. PMID: 21303756
14: Rhabdomyolysis and acute renal failure associated with the co-administration of daptomycin and an HMG-CoA reductase inhibitor.
[Odero Randy O,Cleveland Kerry O,Gelfand Michael S]J Antimicrob Chemother.2009 Jun;63(6):1299-300. doi: 10.1093/jac/dkp127. Epub 2009 Apr 4. PMID: 19346518
15: Daptomycin-induced acute renal and hepatic toxicity without rhabdomyolysis.
[Abraham George,Finkelberg Dmitry,Spooner Linda M]Ann Pharmacother.2008 May;42(5):719-21. doi: 10.1345/aph.1K579. Epub 2008 Apr 1. PMID: 18381844
16: Early-onset rhabdomyolysis related to daptomycin use.
[Patel Samir J,Samo Tobias C,Suki Wadi N]Int J Antimicrob Agents.2007 Nov;30(5):472-4. Epub 2007 Sep 4. PMID: 17804204
17: Rhabdomyolysis during therapy with daptomycin.
[Papadopoulos Stella,Ball Amanda M,Liewer Susanne E,Martin Craig A,Winstead P Shane,Murphy Brian S]Clin Infect Dis.2006 Jun 15;42(12):e108-10. Epub 2006 May 10. PMID: 16705566
18: Rhabdomyolysis and acute renal failure in a patient treated with daptomycin.
[Kazory Amir,Dibadj Kourosh,Weiner I David]J Antimicrob Chemother.2006 Mar;57(3):578-9. Epub 2006 Jan 12. PMID: 16410267
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.