Search for drugs:
Typing the drug name to query
PRAMIPEXOLE DIHYDROCHLORIDE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- The effect of pramipexole on the QT interval of the ECG was investigated in a clinical study in 60 healthy male and female volunteers. All subjects initiated treatment with 0.375 mg pramipexole dihydrochloride extended-release tablets administered once daily, and were up-titrated every 3 days to 2.25 mg and 4.5 mg daily, a faster rate of titration than recommended in the label. No dose- or exposure-related effect on mean QT intervals was observed; however, the study did not have a valid assessment of assay sensitivity. The effect of pramipexole on QTc intervals at higher exposures achieved either due to drug interactions (e.g., with cimetidine), renal impairment, or at higher doses has not been systematically evaluated.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
35
42877
Other ADRs
9397
14107882
Odds Ratio = 1.226
Drug Property Information
ATC Code(s):
- N04BC05 - pramipexole dihydrochloride
- N04BC - Dopamine agonists
- N04B - DOPAMINERGIC AGENTS
- N04 - ANTI-PARKINSON DRUGS
- N - NERVOUS SYSTEM
Active Ingredient:pramipexole dihydrochloride
Active Ingredient UNII:3D867NP06J
Drugbank ID:DB00413
PubChem Compound:119570
CAS Number:104632-26-0
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 2.5 mg/day N04BC05
Chemical Structure: 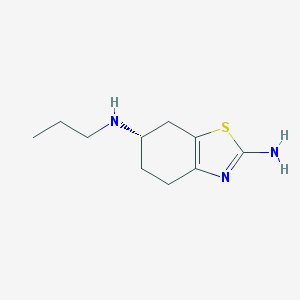

SMILE Code:
CCCN[C@H]1CCC2=C(C1)SC(=N2)N
CCCN[C@H]1CCC2=C(C1)SC(=N2)N
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.