Search for drugs:
Typing the drug name to query
TIPRANAVIR
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CONTRAINDICATIONS
- Drug Interactions
- Co-administration of APTIVUS/ritonavir with drugs that are highly dependent on CYP 3A for clearance or are potent CYP 3A inducers are contraindicated (see Table 1). These recommendations are based on either drug interaction studies or they are predicted interactions due to the expected magnitude of interaction and potential for serious events or loss of efficacy. For information regarding clinical recommendations [see Drug Interactions (7.2)].
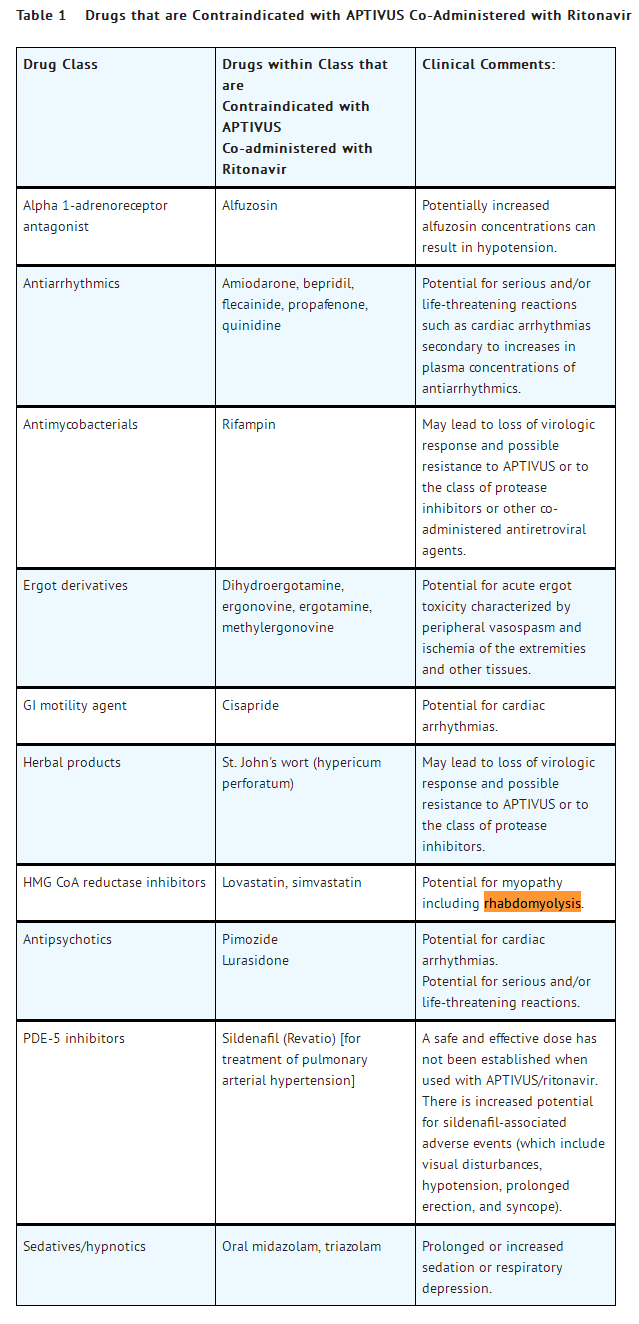
- Due to the need for co-administration of APTIVUS with ritonavir, please refer to the ritonavir prescribing information for a description of ritonavir contraindications.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
1
42911
Other ADRs
865
14116414
Odds Ratio = 0.381
Drug Property Information
ATC Code(s):
- J05AE09 - tipranavir
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:tipranavir
Active Ingredient UNII:ZZT404XD09
Drugbank ID:DB00932
PubChem Compound:54682461
CAS Number:174484-41-4
Dosage Form(s):capsule, liquid filled; solution
Route(s) Of Administrator:oral
Daily Dose:
- 1000.0 mg/day J05AE09
Chemical Structure: 

SMILE Code:
CCC[C@]1(CC(=C(C(=O)O1)[C@H](CC)C2=CC(=CC=C2)NS(=O)(=O)C3=NC=C(C=C3)C(F)(F)F)O)CCC4=CC=CC=C4
CCC[C@]1(CC(=C(C(=O)O1)[C@H](CC)C2=CC(=CC=C2)NS(=O)(=O)C3=NC=C(C=C3)C(F)(F)F)O)CCC4=CC=CC=C4
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.