Search for drugs:
Typing the drug name to query
GABAPENTIN
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing and Other Experience
- In addition to the adverse experiences reported during clinical testing of gabapentin, the following adverse experiences have been reported in patients receiving marketed gabapentin. These adverse experiences have not been listed above and data are insufficient to support an estimate of their incidence or to establish causation. The listing is alphabetized: angioedema, blood glucose fluctuation, breast enlargement, elevated creatine kinase, elevated liver function tests, erythema multiforme, fever, hyponatremia, jaundice, movement disorder, rhabdomyolysis, Stevens-Johnson syndrome.
- Adverse events following the abrupt discontinuation of gabapentin have also been reported. The most frequently reported events were anxiety, insomnia, nausea, pain, and sweating.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
143
42769
Other ADRs
40669
14076610
Odds Ratio = 1.158
Drug Property Information
ATC Code(s):
- N03AX12 - gabapentin
- N03AX - Other antiepileptics
- N03A - ANTIEPILEPTICS
- N03 - ANTIEPILEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:gabapentin
Active Ingredient UNII:6CW7F3G59X
Drugbank ID:DB00996
PubChem Compound:3446
CAS Number:60142-96-3
Dosage Form(s):solution
Route(s) Of Administrator:oral
Daily Dose:
- 1800.0 mg/day N03AX12
Chemical Structure: 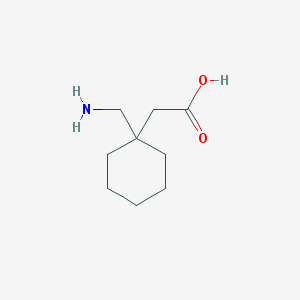

SMILE Code:
C1CCC(CC1)(CC(=O)O)CN
C1CCC(CC1)(CC(=O)O)CN
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Radiologic Findings in Gabapentin-Induced Myositis.
[Coupal Tyler Michael,Chang David Ross,Pennycooke Kevin,Ouellette Hugue Alcide,Munk Peter Loren]J Radiol Case Rep.2017 Apr 30;11(4):30-37. doi: 10.3941/jrcr.v11i4.3092. eCollection 2017 Apr. PMID: 28567183
2: A case of gabapentin-induced rhabdomyolysis requiring renal replacement therapy.
[Choi Min Seok,Jeon Howook,Kim Hyo Suk,Jang Bo Hyun,Lee Yoon Hee,Park Hoon Suk,Kim HyungWook,Jin Dong Chan]Hemodial Int.2017 Jan;21(1):E4-E8. doi: 10.1111/hdi.12458. Epub 2016 Jul 8. PMID: 27389284
3: Trimethoprim-Sulfamethoxazole-Induced Rhabdomyolysis; Gabapentin-Induced Hypoglycemia in Diabetic and Nondiabetic Patients; Purple Glove Syndrome After Oral Phenytoin Administration; Acute Dystonic Reaction After Methylphenidate Initiation; Serotonin Syndrome with Vilazodone Monotherapy; Cabozantinib-Associated Dermatologic Adverse Reactions.
[Mancano Michael A]Hosp Pharm.2015 Sep;50(8):662-6. doi: 10.1310/hpj5008-662. Epub 2015 Sep 16. PMID: 26715798
4: N,N-Dimethyltryptamine-Induced Psychosis.
[Paterson Neil E,Darby W Connor,Sandhu Preetpal S]Clin Neuropharmacol.2015 Jul-Aug;38(4):141-3. doi: 10.1097/WNF.0000000000000078. PMID: 26166234
5: [Rhabdomyolysis from gabapentin: a case report].
[Falconi Daniela,Tattoli Fabio,Brunetti Carlo,De Prisco Ornella,Gherzi Maurizio,Marazzi Federico,Marengo Marita,Serra Ilaria,Tamagnone Michela,Formica Marco]G Ital Nefrol.2015 Mar-Apr;32(2). pii: gin/32.2.37. PMID: 26005946
6: Rhabdomyolysis in a multiple myeloma patient secondary to concurrent treatment with lenalidomide and pravastatin and to lenalidomide alone.
[Shahan Jaime L,Panu Lori D,Hildebrandt Gerhard C]Int J Hematol.2012 Dec;96(6):818-9. doi: 10.1007/s12185-012-1226-3. Epub 2012 Nov 28. PMID: 23188472
7: Gabapentin-induced rhabdomyolysis in a patient with diabetic neuropathy.
[Bilgir Oktay,Calan Mehmet,Bilgir Ferda,Kebapçilar Levent,Yüksel Arif,Yildiz Yasar,Sari Ismail]Intern Med.2009;48(12):1085-7. Epub 2009 Jun 15. PMID: 19525604
8: Gabapentin-induced severe myopathy.
[Tuccori Marco,Lombardo Giuseppe,Lapi Francesco,Vannacci Alfredo,Blandizzi Corrado,Del Tacca Mario]Ann Pharmacother.2007 Jul;41(7):1301-5. Epub 2007 Jun 12. PMID: 17565043
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.