Search for drugs:
Typing the drug name to query
IMATINIB MESYLATE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following additional adverse reactions have been identified during post approval use of imatinib mesylate tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Infections: hepatitis B virus reactivation1
- Nervous System Disorders: cerebral edema1
- Eye Disorders: vitreous hemorrhage
- Cardiac Disorders: pericarditis, cardiac tamponade1
- Vascular Disorders: thrombosis/embolism, anaphylactic shock
- Respiratory, Thoracic and Mediastinal Disorders: acute respiratory failure1, interstitial lung disease
- Gastrointestinal Disorders: ileus/intestinal obstruction, tumor hemorrhage/tumor necrosis, gastrointestinal perforation1 [see Warnings and Precautions (5.6)], diverticulitis, gastric antral vascular ectasia
- Skin and Subcutaneous Tissue Disorders: lichenoid keratosis, lichen planus, toxic epidermal necrolysis, palmar-plantar erythrodysesthesia syndrome, drug rash with eosinophilia and systemic symptoms (DRESS), pseudoporphyria
- Musculoskeletal and Connective Tissue Disorders: avascular necrosis/hip osteonecrosis, rhabdomyolysis/myopathy, growth retardation in children, musculoskeletal pain upon treatment discontinuation (including myalgia, pain in extremity, arthalgia, bone pain)
- Reproduction Disorders: hemorrhagic corpus luteum/hemorrhagic ovarian cyst
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
92
42820
Other ADRs
40334
14076945
Odds Ratio = 0.75
Drug Property Information
ATC Code(s):
- L01XE01 - imatinib mesylate
- L01XE - Protein kinase inhibitors
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:imatinib mesylate
Active Ingredient UNII:8A1O1M485B
Drugbank ID:DB00619
PubChem Compound:5291
CAS Number:152459-95-5
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 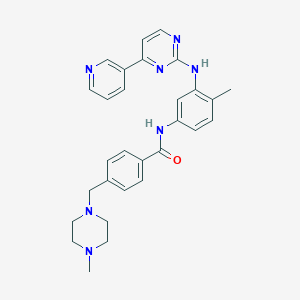

SMILE Code:
CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5
CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [Two cases of surgical resection of rectal gastrointestinal stromal tumor after neoadjuvant therapy with imatinib mesylate].
[Nakashima Susumu,Fujita Yuji,Matsuo Hisataka,Ariyoshi Yosuke,Fukuda Kenichiro,Fujiyama Junshin,Masuyama Mamoru]Gan To Kagaku Ryoho.2012 Nov;39(12):1932-4. PMID: 23267934
2: Sorafenib: muscle wasting.
Prescrire Int.2011 Dec;20(122):296-7. PMID: 22216546
3: Elevations of creatine kinase in patients treated with imatinib mesylate (Gleevec).
[Gordon Jessica K,Magid Steven K,Maki Robert G,Fleisher Martin,Berman Ellin]Leuk Res.2010 Jun;34(6):827-9. doi: 10.1016/j.leukres.2009.11.002. Epub 2009 Dec 5. PMID: 19963273
4: Imatinib as a possible cause of severe rhabdomyolysis.
[Penel Nicolas,Blay Jean-Yves,Adenis Antoine]N Engl J Med.2008 Jun 19;358(25):2746-7. doi: 10.1056/NEJMc0708896. PMID: 18565874
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.