Search for drugs:
Typing the drug name to query
PENICILLING BENZATHINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- As with other penicillins, untoward reactions of the sensitivity phenomena are likely to occur, particularly in individuals who have previously demonstrated hypersensitivity to penicillins or in those with a history of allergy, asthma, hay fever, or urticaria.
- As with other treatments for syphilis, the Jarisch-Herxheimer reaction has been reported.
- The following have been reported with parenteral penicillin G:
- General: Hypersensitivity reactions including the following: skin eruptions (maculopapular to exfoliative dermatitis), urticaria, laryngeal edema, fever, eosinophilia; other serum sickness-like reactions (including chills, fever, edema, arthralgia, and prostration); and anaphylaxis including shock and death. Note: Urticaria, other skin rashes, and serum sickness-like reactions may be controlled with antihistamines and, if necessary, systemic corticosteroids. Whenever such reactions occur, penicillin G should be discontinued unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to therapy with penicillin G. Serious anaphylactic reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management, including intubation, should also be administered as indicated.
- Gastrointestinal: Pseudomembranous colitis. Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment. (See WARNINGS section.)
- Hematologic: Hemolytic anemia, leukopenia, thrombocytopenia.
- Neurologic: Neuropathy.
- Urogenital: Nephropathy.
- The following adverse events have been temporally associated with parenteral administration of penicillin G benzathine:
- Body as a Whole: Hypersensitivity reactions including allergic vasculitis, pruritus, fatigue, asthenia, and pain; aggravation of existing disorder; headache.
- Cardiovascular: Cardiac arrest; hypotension; tachycardia; palpitations; pulmonary hypertension; pulmonary embolism; vasodilation; vasovagal reaction; cerebrovascular accident; syncope.
- Gastrointestinal: Nausea, vomiting; blood in stool; intestinal necrosis.
- Hemic and Lymphatic: Lymphadenopathy.
- Injection Site: Injection site reactions including pain, inflammation, lump, abscess, necrosis, edema, hemorrhage, cellulitis, hypersensitivity, atrophy, ecchymosis, and skin ulcer. Neurovascular reactions including warmth, vasospasm, pallor, mottling, gangrene, numbness of the extremities, cyanosis of the extremities, and neurovascular damage.
- Metabolic: Elevated BUN, creatinine, and SGOT.
- Musculoskeletal: Joint disorder; periostitis; exacerbation of arthritis; myoglobinuria; rhabdomyolysis.
- Nervous System: Nervousness; tremors; dizziness; somnolence; confusion; anxiety; euphoria; transverse myelitis; seizures; coma. A syndrome manifested by a variety of CNS symptoms such as severe agitation with confusion, visual and auditory hallucinations, and a fear of impending death (Hoigne's syndrome), has been reported after administration of penicillin G procaine and, less commonly, after injection of the combination of penicillin G benzathine and penicillin G procaine. Other symptoms associated with this syndrome, such as psychosis, seizures, dizziness, tinnitus, cyanosis, palpitations, tachycardia, and/or abnormal perception in taste, also may occur.
- Respiratory: Hypoxia; apnea; dyspnea.
- Skin: Diaphoresis.
- Special Senses: Blurred vision; blindness.
- Urogenital: Neurogenic bladder; hematuria; proteinuria; renal failure; impotence; priapism.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
0
14117279
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- J01CE08 - penicilling benzathine
- J01CE - Beta-lactamase sensitive penicillins
- J01C - "BETA-LACTAM ANTIBACTERIALS, PENICILLINS"
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:penicillin g benzathine
Active Ingredient UNII:RIT82F58GK
Drugbank ID:DB09323
PubChem Compound:25137901
CAS Number:1538-09-6
Dosage Form(s):injection, suspension
Route(s) Of Administrator:intramuscular
Daily Dose:
Chemical Structure: 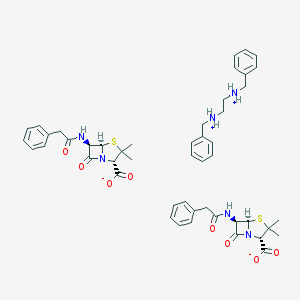

SMILE Code:
CC1([C@@H](N2[C@H](S1)[C@@H](C2=O)NC(=O)CC3=CC=CC=C3)C(=O)[O-])C.CC1([C@@H](N2[C@H](S1)[C@@H](C2=O)NC(=O)CC3=CC=CC=C3)C(=O)[O-])C.C1=CC=C(C=C1)C[NH2+]CC[NH2+]CC2=CC=CC=C2
CC1([C@@H](N2[C@H](S1)[C@@H](C2=O)NC(=O)CC3=CC=CC=C3)C(=O)[O-])C.CC1([C@@H](N2[C@H](S1)[C@@H](C2=O)NC(=O)CC3=CC=CC=C3)C(=O)[O-])C.C1=CC=C(C=C1)C[NH2+]CC[NH2+]CC2=CC=CC=C2
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.