Search for drugs:
Typing the drug name to query
TRAMETINIB
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The heart rate-corrected QT (QTc) prolongation potential of trametinib was assessed in a dedicated study in 32 patients who received placebo on day 1 and MEKINIST 2 mg once daily on days 2-14 followed by MEKINIST 3 mg on day 15. No clinically relevant QTc prolongation was detected in the study.
- In clinical trials in patients who received MEKINIST with dabrafenib, QTc prolongation > 500 ms occurred in 0.8% of patients and QTc increased by > 60 ms from baseline in 3.8% of patients.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
22
42890
Other ADRs
2454
14114825
Odds Ratio = 2.951
Drug Property Information
ATC Code(s):
- L01XE25 - trametinib
- L01XE - Protein kinase inhibitors
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:trametinib dimethyl sulfoxide
Active Ingredient UNII:BSB9VJ5TUT
Drugbank ID:DB08911
PubChem Compound:11707110
CAS Number:871700-17-3
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 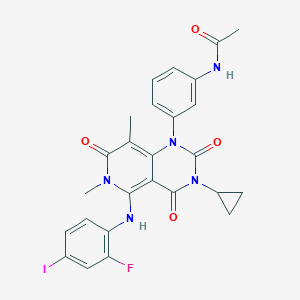

SMILE Code:
CC1=C2C(=C(N(C1=O)C)NC3=C(C=C(C=C3)I)F)C(=O)N(C(=O)N2C4=CC(=CC=C4)NC(=O)C)C5CC5
CC1=C2C(=C(N(C1=O)C)NC3=C(C=C(C=C3)I)F)C(=O)N(C(=O)N2C4=CC(=CC=C4)NC(=O)C)C5CC5
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Success of rechallenging dabrafenib and trametinib combination therapy after trametinib-induced rhabdomyolysis: a case report.
[Muto Yusuke,Ng William,Namikawa Kenjiro,Takahashi Akira,Tsutsumida Arata,Nishida Makiko,Yamazaki Naoya]Melanoma Res.2018 Apr;28(2):151-154. doi: 10.1097/CMR.0000000000000424. PMID: 29356791
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.