Search for drugs:
Typing the drug name to query
CERITINIB
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation
- QTc interval prolongation, which may lead to an increased risk for ventricular tachyarrhythmia (e.g., torsades de pointes) or sudden death, occurred in patients treated with ZYKADIA [see Adverse Reactions (6.1)]. Across clinical studies, 6% of 919 patients with at least one post-baseline electrocardiogram (ECG) assessment had an increase from baseline of QTc > 60 msec. Approximately 1.3% of patients taking ZYKADIA 750 mg under fasted conditions were found to have a QTc > 500 msec. ZYKADIA causes concentration-dependent increases in the QTc interval [see Clinical Pharmacology (12.2)]. Across clinical studies, 0.2% of patients discontinued ZYKADIA due to QTc prolongation.
- When possible, avoid use of ZYKADIA in patients with congenital long QT syndrome. Conduct periodic monitoring with ECGs and electrolytes in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, or those who are taking medications that are known to prolong the QTc interval. Based on the severity of the adverse reaction, withhold ZYKADIA, with resumption at a reduced dose, or permanently discontinue ZYKADIA [see Dosage and Administration (2.3)].
- DRUG INTERACTIONS
- Drugs that Prolong QT Interval
- ZYKADIA causes concentration-dependent increases in the QTc interval. When possible, avoid coadministration of ZYKADIA with other products with a known potential to prolong the QTc interval [see Warnings and Precautions (5.4), Clinical Pharmacology (12.2)].
- DOSAGE AND ADMINISTRATION
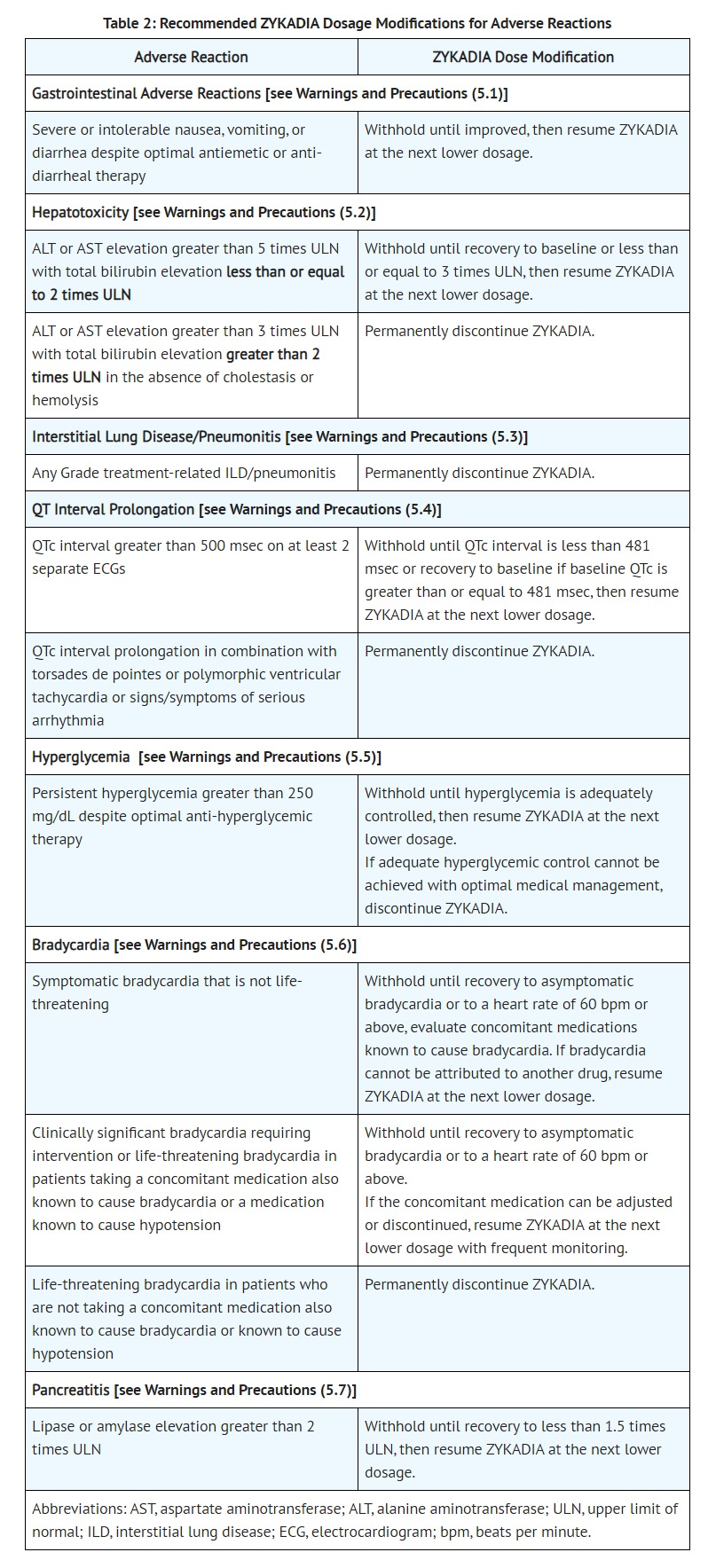
- ADVERSE REACTIONS
- Clinical Trials Experience
- Additional clinically significant adverse reactions occurring in 2% or more of patients treated with ZYKADIA 750 mg under fasted conditions included neuropathy (17% comprised of paresthesia, muscular weakness, gait disturbance, peripheral neuropathy, hypoesthesia, peripheral sensory neuropathy, dysesthesia, neuralgia, peripheral motor neuropathy, hypotonia, or polyneuropathy), vision disorder (9% comprised of vision impairment, blurred vision, photopsia, accommodation disorder, presbyopia, or reduced visual acuity), prolonged QT interval (4%), and bradycardia (3%).
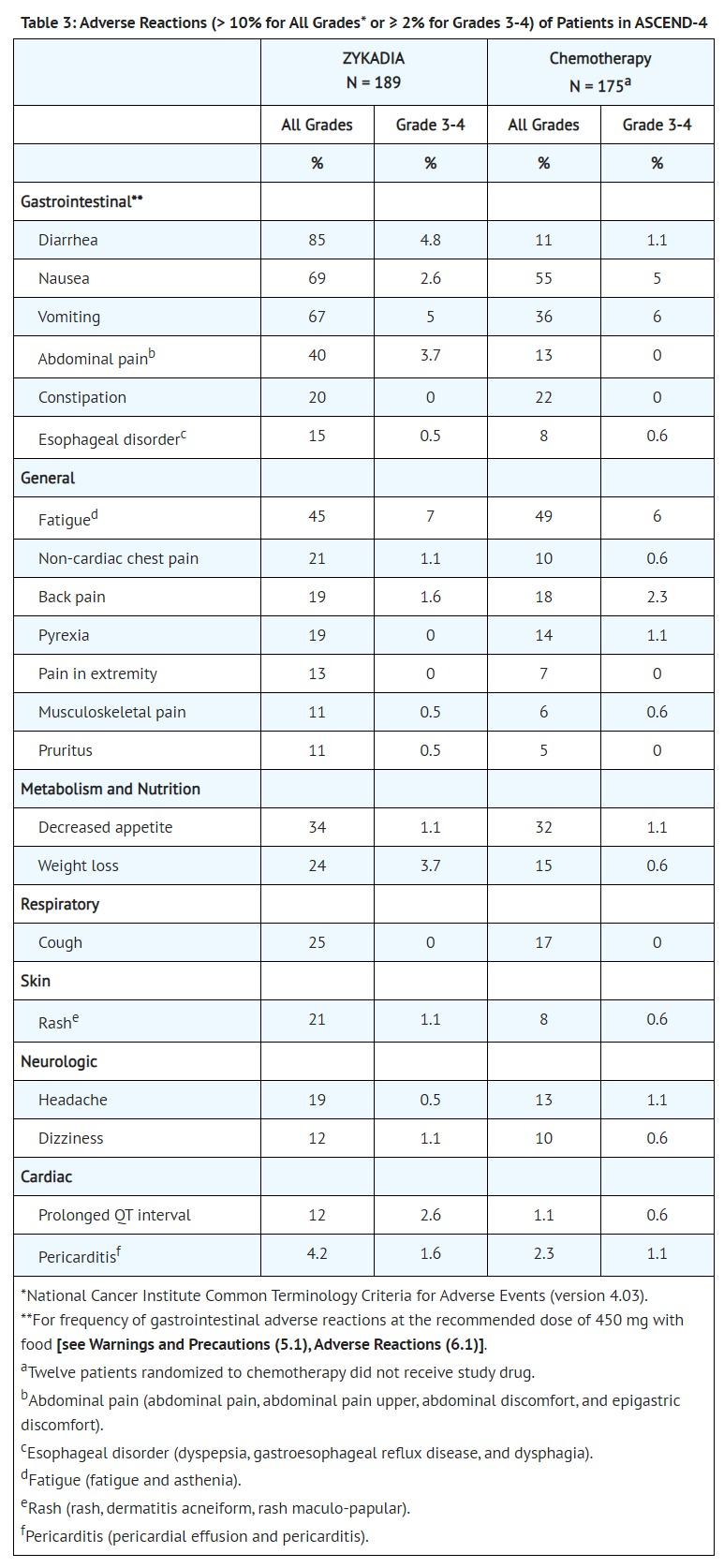
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- Twelve of 919 patients (1.3%) treated with ZYKADIA 750 mg once daily under fasted conditions with at least one post-baseline ECG assessment were found to have a QTc > 500 msec and 58 patients (6%) had an increase from baseline QTc > 60 msec. In ASCEND-4, a central tendency analysis of the QTc data at average steady-state concentrations demonstrated that the upper bound of the 2-sided 90% CI for QTc was 15.3 msec at ZYKADIA 750 mg once daily under fasted conditions. A pharmacokinetic/pharmacodynamic analysis suggested concentration-dependent QTc interval prolongation [see Warnings and Precautions (5.4)].
- Ten of 925 patients (1.1%) had bradycardia defined as < 50 beats/minute [see Warnings and Precautions (5.6)].
- PATIENT COUNSELING INFORMATION
- Arrhythmias
- Inform patients of the risks of QTc interval prolongation and bradycardia. Advise patients to contact their healthcare provider immediately to report new chest pain or discomfort, changes in heartbeat, palpitations, dizziness, lightheadedness, fainting, and changes in or new use of heart or blood pressure medications [see Warnings and Precautions (5.4, 5.6)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
21
24071
Other ADRs
7398
38374189
Odds Ratio = 4.526
Drug Property Information
ATC Code(s):
- L01ED02 - ceritinib
- L01ED -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:CERITINIB
Active Ingredient UNII:K418KG2GET
Drugbank ID:DB09063
PubChem Compound:57379345
CTD ID:C586847
CAS Number:1032900-25-6
Dosage Form(s):capsule; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 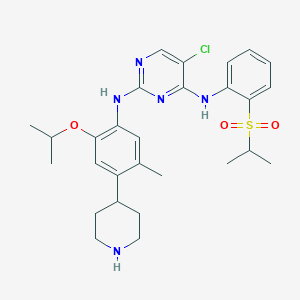

SMILE Code:
CC(C)OC1=C(NC2=NC=C(Cl)C(N2)=NC2=CC=CC=C2S(=O)(=O)C(C)C)C=C(C)C(=C1)C1CCNCC1
CC(C)OC1=C(NC2=NC=C(Cl)C(N2)=NC2=CC=CC=C2S(=O)(=O)C(C)C)C=C(C)C(=C1)C1CCNCC1
Reference
1: Current perspective: Osimertinib-induced QT prolongation: new drugs with new side-effects need careful patient monitoring.
[Schiefer Mart,Hendriks Lizza E L,Dinh Trang,Lalji Ulrich,Dingemans Anne-Marie C]Eur J Cancer,2018 Mar;91:92-98. PMID: 29413968
2: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.