Search for drugs:
Typing the drug name to query
PAROXETINE HYDROCHLORIDE HEMIHYDRATE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Potential Interaction with Thioridazine
- Thioridazine administration alone produces prolongation of the QTc interval, which is associated with serious ventricular arrhythmias, such as torsades de pointes-type arrhythmias, and sudden death. This effect appears to be dose related.
- PRECAUTIONS
- Drug Interactions
- Pimozide
- In a controlled study of healthy volunteers, after paroxetine tablets were titrated to 60 mg daily, co-administration of a single dose of 2 mg pimozide was associated with mean increases in pimozide AUC of 151% and C max of 62%, compared to pimozide administered alone. The increase in pimozide AUC and C max is due to the CYP2D6 inhibitory properties of paroxetine. Due to the narrow therapeutic index of pimozide and its known ability to prolong the QT interval, concomitant use of pimozide and paroxetine tablets are contraindicated (see CONTRAINDICATIONS).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
205
23887
Other ADRs
145053
38236534
Odds Ratio = 2.263
Drug Property Information
ATC Code(s):
- N06AB05 - paroxetine hydrochloride hemihydrate
- N06AB - Selective serotonin reuptake inhibitors
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:PAROXETINE HYDROCHLORIDE HEMIHYDRATE
Active Ingredient UNII:X2ELS050D8
Drugbank ID:DB00715
PubChem Compound:43815
CTD ID:D017374
PharmGKB:PA450801
CAS Number:61869-08-7
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 20.0 mg/day N06AB05
Chemical Structure: 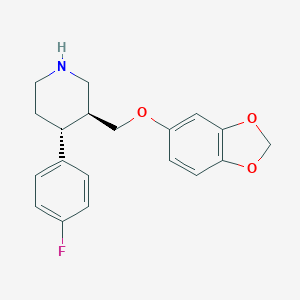

SMILE Code:
FC1=CC=C(C=C1)[C@@H]1CCNC[C@H]1COC1=CC2=C(OCO2)C=C1
FC1=CC=C(C=C1)[C@@H]1CCNC[C@H]1COC1=CC2=C(OCO2)C=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.