Search for drugs:
Typing the drug name to query
DABRAFENIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The potential effect of TAFINLAR on QT interval was assessed in a dedicated multiple-dose study in 32 patients with BRAF V600 mutation-positive tumors. No large changes in the mean QT interval (i.e., > 20 ms) were detected with dabrafenib 300 mg administered twice daily (two times the recommended dosage).
- In clinical trials, QTc (heart rate-corrected QT) prolongation to ≥ 500 ms occurred in 0.8% (2/264) of patients who received TAFINLAR plus trametinib and in 1.5% (4/264) of patients who received TAFINLAR as a single agent. The QTc was increased > 60 ms from baseline in 3.8% (10/264) of patients who received TAFINLAR plus trametinib and 3% (8/264) of patients treated with TAFINLAR as a single agent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
39
24053
Other ADRs
25157
38356430
Odds Ratio = 2.473
Drug Property Information
ATC Code(s):
- L01EC02 - dabrafenib
- L01EC -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:DABRAFENIB MESYLATE
Active Ingredient UNII:B6DC89I63E
Drugbank ID:DB08912
PubChem Compound:44462760
CTD ID: C561627
CAS Number:1195765-45-7
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 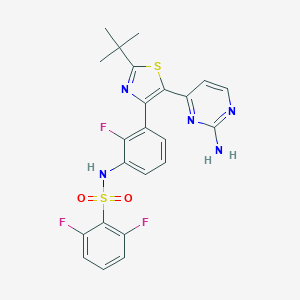

SMILE Code:
CC(C)(C)C1=NC(=C(S1)C1=NC(N)=NC=C1)C1=C(F)C(NS(=O)(=O)C2=C(F)C=CC=C2F)=CC=C1
CC(C)(C)C1=NC(=C(S1)C1=NC(N)=NC=C1)C1=C(F)C(NS(=O)(=O)C2=C(F)C=CC=C2F)=CC=C1
Reference
1: Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis.
[Mincu Raluca I,Mahabadi Amir A,Michel Lars,Mrotzek Simone M,Schadendorf Dirk,Rassaf Tienush,Totzeck Matthias]JAMA Netw Open,2019 Aug 2;2(8):e198890. PMID: 31397860
2: Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management.
[Heinzerling Lucie,Eigentler Thomas K,Fluck Michael,Hassel Jessica C,Heller-Schenck Daniela,Leipe Jan,Pauschinger Matthias,Vogel Arndt,Zimmer Lisa,Gutzmer Ralf]ESMO Open,2019 May 23;4(3):e000491. PMID: 31231568
3: Evaluation of the effect of dabrafenib and metabolites on QTc interval in patients with BRAF V600-mutant tumours.
[Nebot Noelia,Arkenau Hendrik-Tobias,Infante Jeffrey R,Chandler Jason C,Weickhardt Andrew,Lickliter Jason D,Sarantopoulos John,Gordon Michael S,Mak Gabriel,St-Pierre Annie,Tang Lihua,Mookerjee Bijoyesh,Carson Stanley W,Hayes Siobhan,Grossmann Kenneth F]Br J Clin Pharmacol,2018 Apr;84(4):764-775. PMID: 29243287
4: What links BRAF to the heart function? New insights from the cardiotoxicity of BRAF inhibitors in cancer treatment.
[Bronte Enrico,Bronte Giuseppe,Novo Giuseppina,Bronte Fabrizio,Bavetta Maria Grazia,Lo Re Giuseppe,Brancatelli Giuseppe,Bazan Viviana,Natoli Clara,Novo Salvatore,Russo Antonio]Oncotarget,2015 Nov 3;6(34):35589-601. PMID: 26431495
5: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.