Search for drugs:
Typing the drug name to query
TIGECYCLINE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- No significant effect of a single intravenous dose of TYGACIL 50 mg or 200 mg on QTc interval was detected in a randomized, placebo- and active-controlled four-arm crossover thorough QTc study of 46 healthy subjects.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
3
24089
Other ADRs
4089
38377498
Odds Ratio = 1.169
Drug Property Information
ATC Code(s):
- J01AA12 - tigecycline
- J01AA - Tetracyclines
- J01A - TETRACYCLINES
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:TIGECYCLINE
Active Ingredient UNII:70JE2N95KR
Drugbank ID:DB00560
PubChem Compound:54686904
CTD ID: D000078304
PharmGKB:PA164746412
CAS Number:220620-09-7
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 100.0 mg/day J01AA12
Chemical Structure: 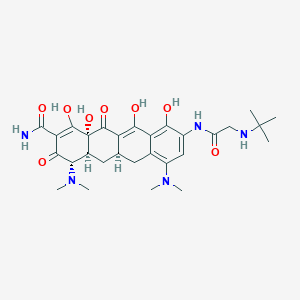

SMILE Code:
[H][C@@]12CC3=C(C(O)=C(NC(=O)CNC(C)(C)C)C=C3N(C)C)C(=O)C1=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@@H](N(C)C)[C@]1([H])C2
[H][C@@]12CC3=C(C(O)=C(NC(=O)CNC(C)(C)C)C=C3N(C)C)C(=O)C1=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@@H](N(C)C)[C@]1([H])C2
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.