Search for drugs:
Typing the drug name to query
AFATINIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of multiple doses of GILOTRIF (50 mg once daily) on the QTc interval was evaluated in an open-label, single-arm study in patients with relapsed or refractory solid tumors. No large changes in the mean QTc interval (i.e., >20 ms) were detected in the study.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
19010
38362577
Odds Ratio = 0.084
Drug Property Information
ATC Code(s):
- L01EB03 - afatinib
- L01EB -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:AFATINIB
Active Ingredient UNII:41UD74L59M
Drugbank ID:DB08916
PubChem Compound:10184653
CTD ID:D000077716
PharmGKB:PA165981154
CAS Number:850140-72-6
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 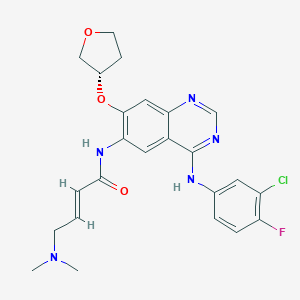

SMILE Code:
CN(C)C\C=C\C(=O)NC1=C(O[C@H]2CCOC2)C=C2N=CN=C(NC3=CC(Cl)=C(F)C=C3)C2=C1
CN(C)C\C=C\C(=O)NC1=C(O[C@H]2CCOC2)C=C2N=CN=C(NC3=CC(Cl)=C(F)C=C3)C2=C1
Reference
1: Osimertinib induced cardiomyopathy: A case report.
[Shinomiya Shun,Kaira Kyoichi,Yamaguchi Ou,Ishikawa Keitaro,Kagamu Hiroshi]Medicine (Baltimore),2020 Sep 25;99(39):e22301. PMID: 32991436
2: QT interval prolongation related to afatinib treatment in a patient with metastatic non-small-cell lung cancer.
[Demircan Nazım Can,Akın Telli Tuğba,Başoğlu Tüylü Tuğba,Arıkan Rukiye,Kocakaya Derya,Şahin Ahmet Anıl,Ercelep Özlem,Dane Faysal,Yumuk Perran Fulden]Curr Probl Cancer,2020 Dec;44(6):100594. PMID: 32505368
3: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.