Search for drugs:
Typing the drug name to query
PERPHENAZINE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- In the event of overdosage, emergency treatment should be started immediately. Consultation with a poison center should be considered. All patients suspected of having taken an overdose should be hospitalized as soon as possible.
- [Manifestations]
- The toxic effects of perphenazine are typically mild to moderate with death occurring in cases involving a large overdose. Overdosage of perphenazine primarily involves the extrapyramidal mechanism and produces the same side effects described under ADVERSE REACTIONS, but to a more marked degree. It is usually evidenced by stupor or coma; children may have convulsive seizures. Signs of arousal may not occur for 48 hours. The primary effects of medical concern are cardiac in origin including tachycardia, prolongation of the QRS or QTc intervals, atrioventricular block, torsade de pointes, ventricular dysrhythmia, hypotension or cardiac arrest, which indicate serious poisoning. Deaths by deliberate or accidental overdosage have occurred with this class of drugs.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
832
38380755
Odds Ratio = 1.915
Drug Property Information
ATC Code(s):
- N05AB03 - perphenazine
- N05AB - Phenothiazines with piperazine structure
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:PERPHENAZINE
Active Ingredient UNII:FTA7XXY4EZ
Drugbank ID:DB00850
PubChem Compound:4748
CTD ID:D010546
PharmGKB:PA450882
CAS Number:58-39-9
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 30.0 mg/day N05AB03
Chemical Structure: 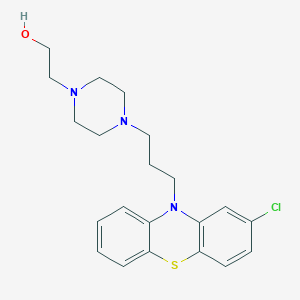

SMILE Code:
OCCN1CCN(CCCN2C3=CC=CC=C3SC3=C2C=C(Cl)C=C3)CC1
OCCN1CCN(CCCN2C3=CC=CC=C3SC3=C2C=C(Cl)C=C3)CC1
Reference
1: Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.
[Weibel Stephanie,Rücker Gerta,Eberhart Leopold Hj,Pace Nathan L,Hartl Hannah M,Jordan Olivia L,Mayer Debora,Riemer Manuel,Schaefer Maximilian S,Raj Diana,Backhaus Insa,Helf Antonia,Schlesinger Tobias,Kienbaum Peter,Kranke Peter]Cochrane Database Syst Rev,2020 Oct 19;10:CD012859. PMID: 33075160
2: Antipsychotics and the Risks of Sudden Cardiac Death and All-Cause Death: Cohort Studies in Medicaid and Dually-Eligible Medicaid-Medicare Beneficiaries of Five States.
[Leonard Charles E,Freeman Cristin P,Newcomb Craig W,Bilker Warren B,Kimmel Stephen E,Strom Brian L,Hennessy Sean]J Clin Exp Cardiolog,2013;Suppl 10(6):1-9. PMID: 24027655
3: Antipsychotic drugs and QT prolongation.
[Stöllberger Claudia,Huber Johannes O,Finsterer Josef]Int Clin Psychopharmacol,2005 Sep;20(5):243-51. PMID: 16096514
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.