Search for drugs:
Typing the drug name to query
DELAFLOXACIN MEGLUMINE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, positive- and placebo-controlled, thorough QT/QTc study, 51 healthy subjects received BAXDELA 300 mg IV, BAXDELA 900 mg IV, oral moxifloxacin 400 mg, or placebo. Neither BAXDELA 300 mg nor BAXDELA 900 mg (three times the intravenous therapeutic dose) had any clinically relevant adverse effect on cardiac repolarization.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
397
38381190
Odds Ratio = 4.014
Drug Property Information
ATC Code(s):
- J01MA23 - delafloxacin meglumine
- J01MA - Fluoroquinolones
- J01M - QUINOLONE ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:delafloxacin meglumine
Active Ingredient UNII:N7V53U4U4T
Drugbank ID:DB11943
PubChem Compound:487101
CTD ID: C477891
CAS Number:189279-58-1
Dosage Form(s):injection, powder, lyophilized, for solution; tablet
Route(s) Of Administrator:intravenous; oral
Daily Dose:
- 900.0 mg/day J01MA23
Chemical Structure: 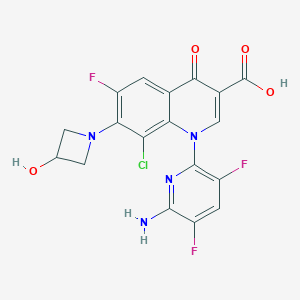

SMILE Code:
NC1=NC(N2C=C(C(O)=O)C(=O)C3=CC(F)=C(N4CC(O)C4)C(Cl)=C23)=C(F)C=C1F
NC1=NC(N2C=C(C(O)=O)C(=O)C3=CC(F)=C(N4CC(O)C4)C(Cl)=C23)=C(F)C=C1F
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.