Search for drugs:
Typing the drug name to query
OXYBUTYNIN CHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Surveillance
- Because postmarketing adverse events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following additional adverse events have been reported from worldwide postmarketing experience with oral oxybutynin chloride: Psychiatric Disorders: psychotic disorder, agitation, hallucination, memory impairment; Nervous System Disorders: convulsions; Eye Disorders: cycloplegia, mydriasis, glaucoma; Cardiac Disorders: tachycardia, QT interval prolongation, chest discomfort; Gastrointestinal Disorders: decreased gastrointestinal motility, frequent bowel movements; Skin and Subcutaneous Tissue Disorders: rash, decreased sweating; Renal and Urinary Disorders: impotence; Reproductive System and Breast Disorders: suppression of lactation; General Disorders and Administration Site Conditions: hypersensitivity reactions, including angioedema with airway obstruction, urticaria, and face edema; rare anaphylactic reactions requiring hospitalization for emergency treatment; Metabolism and Nutrition Disorders: anorexia; Respiratory, Thoracic and Mediastinal Disorders: dysphonia.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
2
24090
Other ADRs
20036
38361551
Odds Ratio = 0.159
Drug Property Information
ATC Code(s):
- G04BD04 - oxybutynin chloride
- G04BD - Urinary antispasmodics
- G04B - "OTHER UROLOGICALS, INCL. ANTISPASMODICS"
- G04 - UROLOGICALS
- G - GENITO URINARY SYSTEM AND SEX HORMONES
Active Ingredient:OXYBUTYNIN CHLORIDE
Active Ingredient UNII:L9F3D9RENQ
Drugbank ID:DB01062
PubChem Compound:4634
CTD ID:C005419
PharmGKB:PA164746030
CAS Number:5633-20-5
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 1.5 mg/day G04BD04
Chemical Structure: 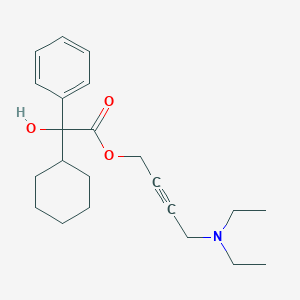

SMILE Code:
CCN(CC)CC#CCOC(=O)C(O)(C1CCCCC1)C1=CC=CC=C1
CCN(CC)CC#CCOC(=O)C(O)(C1CCCCC1)C1=CC=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.