Search for drugs:
Typing the drug name to query
LENVATINIB
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Renal Failure or Impairment
- Serious including fatal renal failure or impairment can occur with LENVIMA. Renal impairment occurred in 14% of patients receiving LENVIMA in SELECT (DTC) and in 7% of patients receiving LENVIMA in REFLECT (HCC). Grade 3 to 5 renal failure or impairment occurred in 3% (DTC) and 2% (HCC) of patients, including 1 fatality in each study.
- In Study 205 (RCC), renal impairment or renal failure occurred in 18% of patients receiving LENVIMA with everolimus, including Grade 3 in 10% of patients [see Adverse Reactions (6.1)].
- Initiate prompt management of diarrhea or dehydration/hypovolemia. Withhold and resume at a reduced dose upon recovery or permanently discontinue LENVIMA for renal failure or impairment based on severity [see Dosage and Administration (2.6)].
- [QT Interval Prolongation]
- In SELECT (DTC), QT/QTc interval prolongation occurred in 9% of LENVIMA-treated patients and QT interval prolongation of >500 ms occurred in 2%. In Study 205 (RCC), QTc interval increases of >60 ms occurred in 11% of patients receiving LENVIMA with everolimus and QTc interval >500 ms occurred in 6%. In REFLECT (HCC), QTc interval increases of >60 ms occurred in 8% of LENVIMA-treated patients and QTc interval >500 ms occurred in 2%.
- Monitor and correct electrolyte abnormalities at baseline and periodically during treatment. Monitor electrocardiograms in patients with congenital long QT syndrome, congestive heart failure, bradyarrhythmias, or those who are taking drugs known to prolong the QT interval, including Class Ia and III antiarrhythmics. Withhold and resume at reduced dose of LENVIMA upon recovery based on severity [see Dosage and Administration (2.6)].
- DRUG INTERACTIONS
- Drugs That Prolong the QT Interval
- LENVIMA has been reported to prolong the QT/QTc interval. Avoid coadministration of LENVIMA with medicinal products with a known potential to prolong the QT/QTc interval [see Warnings and Precautions (5.9)].
- DOSAGE AND ADMINISTRATION
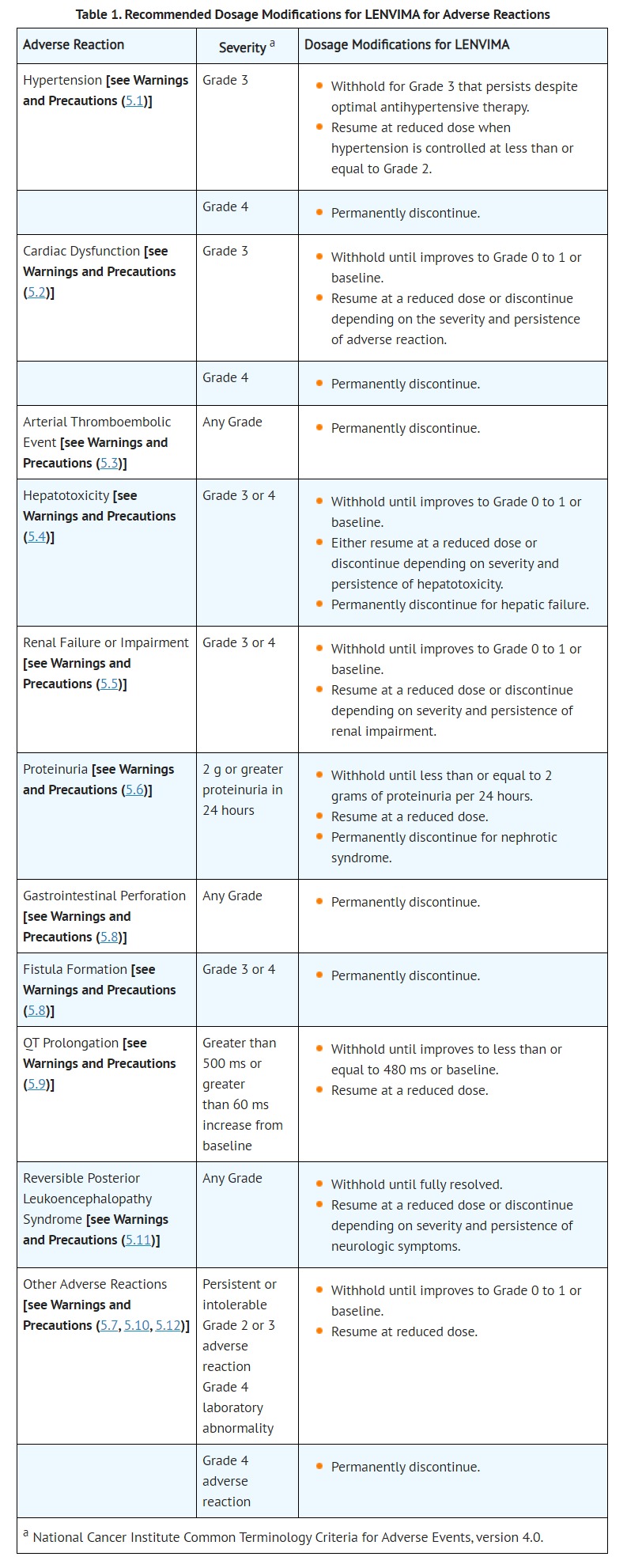
- ADVERSE REACTIONS
- The following adverse reactions are discussed elsewhere in the labeling:
- Renal Failure and Impairment [see Warnings and Precautions (5.5)]
- QT Interval Prolongation [see Warnings and Precautions (5.9)]
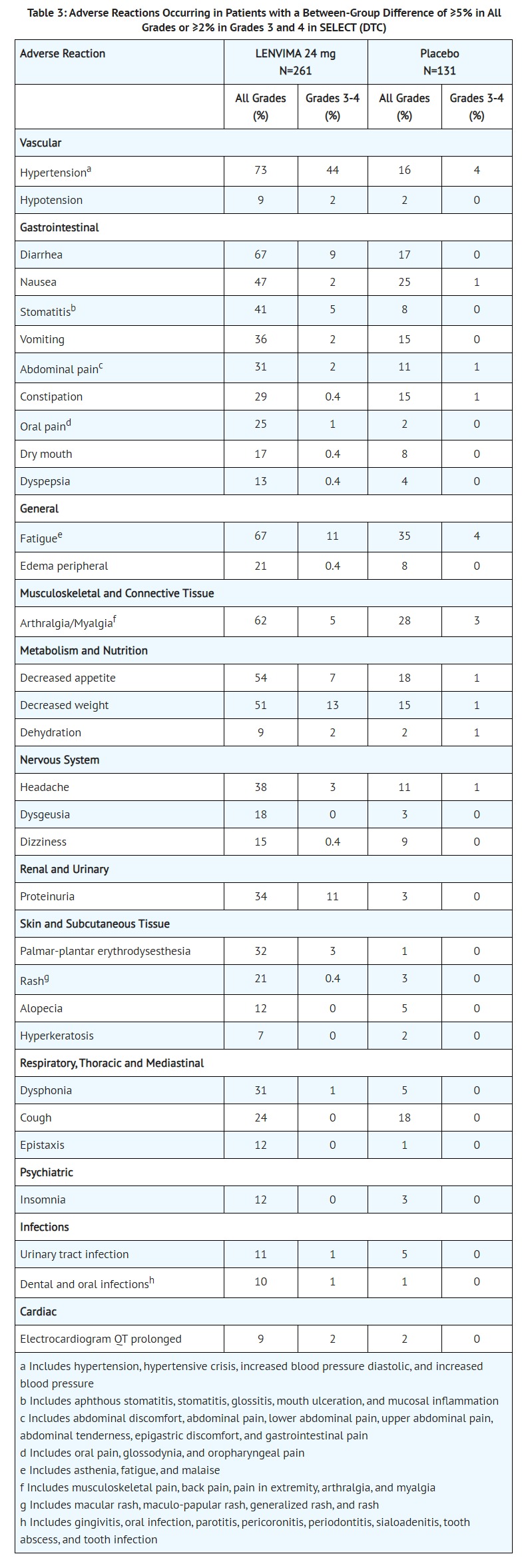
- PATIENT COUNSELING INFORMATION
- QTc Interval Prolongation
- Advise patients who are at risk for QTc prolongation that they will need to undergo regular ECGs. Advise all patients that they will need to undergo laboratory tests to monitor electrolytes [see Warnings and Precautions (5.9)].
- PATIENT PACKAGE INSERT
- changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life threatening. Your healthcare provider will do blood tests before and during your treatment with LENVIMA to check the levels of potassium, magnesium, and calcium in your blood, and may check the electrical activity of your heart with an ECG.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
28
24064
Other ADRs
33986
38347601
Odds Ratio = 1.313
Drug Property Information
ATC Code(s):
- L01EX08 - lenvatinib
- L01EX0 -
- L01EX -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:LENVATINIB
Active Ingredient UNII:EE083865G2
Drugbank ID:DB09078
PubChem Compound:9823820
CTD ID: C531958
PharmGKB:PA166153472
CAS Number:417716-92-8
Dosage Form(s):capsule; kit
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 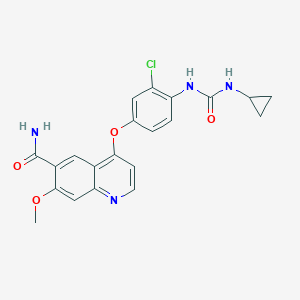

SMILE Code:
COC1=C(C=C2C(OC3=CC=C(NC(=O)NC4CC4)C(Cl)=C3)=CC=NC2=C1)C(N)=O
COC1=C(C=C2C(OC3=CC=C(NC(=O)NC4CC4)C(Cl)=C3)=CC=NC2=C1)C(N)=O
Reference
1: Comparative efficacy and safety of tyrosine kinase inhibitors for thyroid cancer: a systematic review and meta-analysis.
[Oba Takaaki,Chino Tatsunori,Soma Ai,Shimizu Tadafumi,Ono Mayu,Ito Tokiko,Kanai Toshiharu,Maeno Kazuma,Ito Ken-Ichi]Endocr J,2020 Dec 28;67(12):1215-1226. PMID: 32814730
2: Safety of Tyrosine Kinase Inhibitors in Patients With Differentiated Thyroid Cancer: Real-World Use of Lenvatinib and Sorafenib in Korea.
[Kim Soo Young,Kim Seok-Mo,Chang Hojin,Kim Bup-Woo,Lee Yong Sang,Chang Hang-Seok,Park Cheong Soo]Front Endocrinol (Lausanne),2019 Jun 12;10:384. PMID: 31244783
3: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
4: Effect of lenvatinib (E7080) on the QTc interval: results from a thorough QT study in healthy volunteers.
[Shumaker Robert C,Zhou Meijian,Ren Min,Fan Jean,Martinez Gresel,Aluri Jagadeesh,Darpo Borje]Cancer Chemother Pharmacol,2014 Jun;73(6):1109-17. PMID: 24658627
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.