Search for drugs:
Typing the drug name to query
PRAMIPEXOLE DIHYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- The effect of pramipexole on the QT interval of the ECG was investigated in a clinical study in 60 healthy male and female volunteers. All subjects initiated treatment with 0.375 mg pramipexole dihydrochloride extended-release tablets administered once daily, and were up-titrated every 3 days to 2.25 mg and 4.5 mg daily, a faster rate of titration than recommended in the label. No dose- or exposure-related effect on mean QT intervals was observed; however, the study did not have a valid assessment of assay sensitivity. The effect of pramipexole on QTc intervals at higher exposures achieved either due to drug interactions (e.g., with cimetidine), renal impairment, or at higher doses has not been systematically evaluated.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
12
24080
Other ADRs
26144
38355443
Odds Ratio = 0.732
Drug Property Information
ATC Code(s):
- N04BC05 - pramipexole dihydrochloride
- N04BC - Dopamine agonists
- N04B - DOPAMINERGIC AGENTS
- N04 - ANTI-PARKINSON DRUGS
- N - NERVOUS SYSTEM
Active Ingredient:PRAMIPEXOLE DIHYDROCHLORIDE
Active Ingredient UNII:3D867NP06J
Drugbank ID:DB00413
PubChem Compound:119570
CTD ID:D000077487
PharmGKB:PA164742949
CAS Number:104632-26-0
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 2.5 mg/day N04BC05
Chemical Structure: 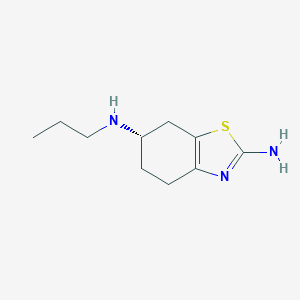

SMILE Code:
CCCN[C@H]1CCC2=C(C1)SC(N)=N2
CCCN[C@H]1CCC2=C(C1)SC(N)=N2
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.