Search for drugs:
Typing the drug name to query
OSILODROSTAT
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Prolongation
- ISTURISA is associated with a dose-dependent QT interval prolongation (maximum mean estimated QTcF increase of up to 5.3 ms at 30 mg), which may cause cardiac arrhythmias [see Adverse Reactions ( 6), Clinical Pharmacology ( 12.2)] .
- Perform an ECG to obtain a baseline QTc interval measurement prior to initiating therapy with ISTURISA and monitor for an effect on the QTc interval thereafter. Correct hypokalemia and/or hypomagnesemia prior to ISTURISA initiation and monitor periodically during treatment with ISTURISA. Correct electrolyte abnormalities if indicated. Consider temporary discontinuation of ISTURISA in the case of an increase in QTc interval > 480 ms.
- Use caution in patients with risk factors for QT prolongation, (such as congenital long QT syndrome, congestive heart failure, bradyarrythmias, uncorrected electrolyte abnormalities, and concomitant medications known to prolong the QT interval) and consider more frequent ECG monitoring.
- OVERDOSAGE
- In case of suspected overdosage, ISTURISA should be temporarily discontinued, cortisol levels should be checked, and if necessary, corticosteroid supplementation should be initiated. Close surveillance may be necessary, including monitoring of the QT interval, blood pressure, glucose, fluid, and electrolyte until the patient’s condition is stable.
- ADVERSE REACTIONS
- Other notable adverse reactions which occurred with a frequency less than 10% were: hirsutism (9.5%), acne (8.8%), dyspepsia (8%), insomnia (8%), anxiety (7.3%), depression (7.3%), gastroenteritis (7.3%), malaise (6.6%), tachycardia (6.6%), alopecia (5.8%), transaminases increased (4.4%), electrocardiogram QT prolongation (3.6%), and syncope (1.5%).
- [QTc Interval Prolongation]
- Adverse reactions of QT prolongation and clinically relevant ECG findings were reported. Five (4%) patients had an event of QT prolongation, 3 (2%) patients had a QTcF increase of > 60 ms from baseline, and 18 (13%) had a new QTcF value of > 450 ms [see Clinical Pharmacology ( 12.2)] .
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- A thorough QT study in 86 male and female healthy volunteers showed a maximum mean placebo-corrected QTcF interval increase of 1.73 ms [90% confidence interval (CI): 0.15, 3.31] at a 10 mg dose, and 25.38 ms (90% CI: 23.53, 27.22) at a 150 mg dose (up to 2.5 times the maximum recommended dosage) [see Warnings and Precautions ( 5.2)] .
- The predicted mean placebo-corrected QTcF change from baseline at the highest recommended dose in clinical practice (30 mg twice daily) was estimated as 5.3 ms (90% CI: 4.2, 6.5), based on an interpolation of the data from the thorough QT Study and population PK analysis [see Warnings and Precautions ( 5.2)] .
- PATIENT COUNSELING INFORMATION
- QT Prolongation
- Advise patients of the signs and symptoms of QT prolongation. Advise patients to contact their healthcare provider immediately for signs or symptoms of QT prolongation.
- Advise patients that an ECG will be taken before treatment and periodically thereafter. Advise patients with cardiac disease and risk factors for QT prolongation that adjustments in cardiac medications may be made and electrolyte disturbances may require correction [see Warnings and Precautions ( 5.2)] .
- PATIENT PACKAGE INSERT
- Heart problem or a heart rhythm problem, such as an irregular heartbeat which could be a sign of a heart problem called QT prolongation. Call your healthcare provider right away if you have irregular heartbeats.
- Before you take ISTURISA, tell your healthcare provider about all of your medical conditions, including if you:
- have or had heart problems, such as an irregular heartbeat, including a condition called prolonged QT syndrome (QT internal prolongation). Your healthcare provider will check the electrical signal of your heart (called an electrocardiogram) before you start taking ISTURISA, 1 week after starting ISTURISA, and as needed after that.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- H02CA02 - osilodrostat
- H02CA - Anticorticosteroids
- H02C - ANTIADRENAL PREPARATIONS
- H02 - CORTICOSTEROIDS FOR SYSTEMIC USE
- H - "SYSTEMIC HORMONAL PREPARATIONS, EXCL. "
Active Ingredient:OSILODROSTAT PHOSPHATE
Active Ingredient UNII:Y6581YAW9V
Drugbank ID:DB11837
PubChem Compound:44139752
CTD ID: C553306
CAS Number:928134-65-0
Dosage Form(s):tablet, coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 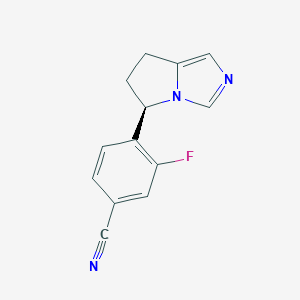

SMILE Code:
FC1=C(C=CC(=C1)C#N)[C@H]1CCC2=CN=CN12
FC1=C(C=CC(=C1)C#N)[C@H]1CCC2=CN=CN12
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.