Search for drugs:
Typing the drug name to query
MOXIFLOXACIN HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- Moxifloxacin hydrochloride has been shown to prolong the QT interval of the electrocardiogram in some patients. Following oral dosing with 400 mg of moxifloxacin hydrochloride the mean (± SD) change in QTc from the pre-dose value at the time of maximum drug concentration was 6 msec (± 26) (n = 787). Following a course of daily intravenous dosing (400 mg; 1 hour infusion each day) the mean change in QTc from the Day 1 pre-dose value was 10 msec (±22) on Day 1 (n=667) and 7 msec (± 24) on Day 3 (n = 667).
- Avoid moxifloxacin hydrochloride in patients with the following risk factors due to the lack of clinical experience with the drug in these patient populations:
- Known prolongation of the QT interval
- Ventricular arrhythmias including torsade de pointes because QT prolongation may lead to an increased risk for these conditions
- Ongoing proarrhythmic conditions, such as clinically significant bradycardia and acute myocardial ischemia,
- Uncorrected hypokalemia or hypomagnesemia
- Class IA (for example, quinidine, procainamide) or Class III (for example, amiodarone, sotalol) antiarrhythmic agents
- Other drugs that prolong the QT interval such as cisapride, erythromycin, antipsychotics, and tricyclic antidepressants
- Elderly patients using intravenous moxifloxacin hydrochloride may be more susceptible to drug-associated QT prolongation [see USE IN SPECIFIC POPULATIONS (8.5)].
- In patients with mild, moderate, or severe liver cirrhosis, metabolic disturbances associated with hepatic insufficiency may lead to QT prolongation. Monitor ECG in patients with liver cirrhosis treated with moxifloxacin hydrochloride [see CLINICAL PHARMACOLOGY (12.3)].
- The magnitude of QT prolongation may increase with increasing concentrations of the drug or increasing rates of infusion of the intravenous formulation. Therefore, the recommended dose or infusion rate should not be exceeded.
- In premarketing clinical trials, the rate of cardiovascular adverse reactions was similar in 798 moxifloxacin hydrochloride and 702 comparator treated patients who received concomitant therapy with drugs known to prolong the QTc interval. No excess in cardiovascular morbidity or mortality attributable to QTc prolongation occurred with moxifloxacin hydrochloride treatment in over 15,500 patients in controlled clinical studies, including 759 patients who were hypokalemic at the start of treatment, and there was no increase in mortality in over 18,000 moxifloxacin tablet treated patients in a postmarketing observational study in which ECGs were not performed.
- DRUG INTERACTIONS
- Drugs that Prolong QT
- There is limited information available on the potential for a pharmacodynamic interaction in humans between moxifloxacin hydrochloride and other drugs that prolong the QTc interval of the electrocardiogram. Sotalol, a Class III antiarrhythmic, has been shown to further increase the QTc interval when combined with high doses of intravenous moxifloxacin hydrochloride in dogs. Therefore, moxifloxacin hydrochloride should be avoided with Class IA and Class III antiarrhythmics [see WARNINGS AND PRECAUTIONS (5.6), and NONCLINICAL TOXICOLOGY (13.2)].
- OVERDOSAGE
- Single oral overdoses up to 2.8 g were not associated with any serious adverse events. In the event of acute overdose, empty the stomach and maintain adequate hydration. Monitor ECG due to the possibility of QT interval prolongation. Carefully observe the patient and give supportive treatment. The administration of activated charcoal as soon as possible after oral overdose may prevent excessive increase of systemic moxifloxacin exposure. About 3% and 9% of the dose of moxifloxacin, as well as about 2% and 4.5% of its glucuronide metabolite are removed by continuous ambulatory peritoneal dialysis and hemodialysis, respectively.
- ADVERSE REACTIONS
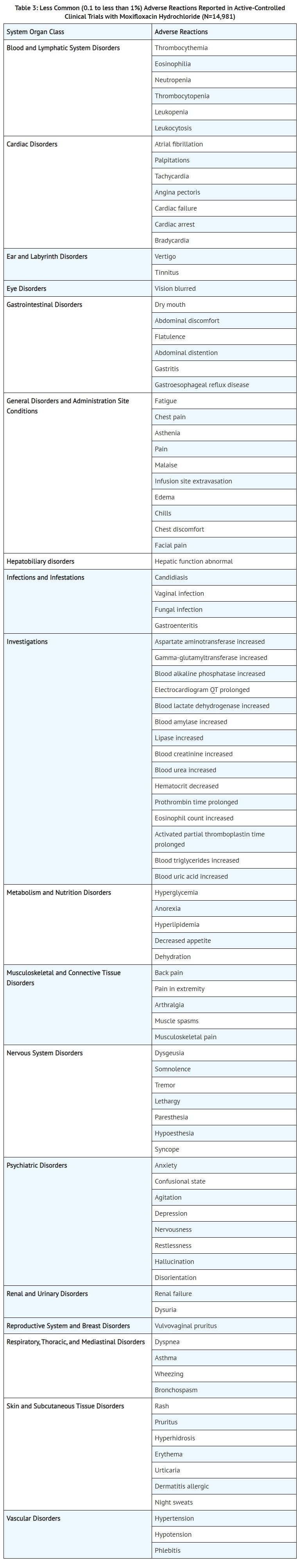
- CLINICAL PHARMACOLOGY
- Pharmacokinetics
- Hepatic Insufficiency
- No dosage adjustment is recommended for mild, moderate, or severe hepatic insufficiency (Child-Pugh Classes A, B, or C). However, due to metabolic disturbances associated with hepatic insufficiency, which may lead to QT prolongation, moxifloxacin hydrochloride should be used with caution in these patients [see WARNINGS AND PRECAUTIONS (5.6) and USE IN SPECIFIC POPULATIONS (8.7)].
- PATIENT COUNSELING INFORMATION
- Serious Adverse Reactions
- Prolongation of the QT Interval: Instruct patients to inform their physician of any personal or family history of QT prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia; if they are taking any Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents. Instruct patients to notify their physician if they have any symptoms of prolongation of the QT interval, including prolonged heart palpitations or a loss of consciousness.
- MEDICATION GUIDE
- Moxifloxacin tablets can cause side effects that may be serious or even cause death, including:
- Serious heart rhythm changes (QT prolongation and torsade de pointes). Tell your healthcare provider right away if you have a change in your heartbeat (a fast or irregular heartbeat), or if you faint. Moxifloxacin tablets may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this event are higher in people:
- Who are elderly
- With a family history of prolonged QT interval
- With low blood potassium (hypokalemia)
- Who take certain medicines to control heart rhythm (antiarrhythmics)
- USE IN SPECIFIC POPULATIONS
- Pediatric Use
- The overall adverse reaction profile in pediatric patients was comparable to that of adult patients. The most frequently occurring adverse reactions in pediatric patients treated with moxifloxacin hydrochloride were QT prolongation 9.3% (28/301), vomiting, 6.6% (20/301), diarrhea 3.7% (11/301), arthralgia 3.0% (9/301), and phlebitis 2.7% (8/301) (see Table 5). Discontinuation of study drug due to an adverse reaction was reported in 5.3% (16/301) of moxifloxacin hydrochloride-treated patients versus 1.3% (2/150) of comparator-treated patients. The adverse reaction profile of moxifloxacin hydrochloride or comparator was similar across all age groups studied.
- [Geriatric Use]
- In trials of intravenous use, 42% of moxifloxacin hydrochloride patients were greater than or equal to 65 years of age, and 23% were greater than or equal to 75 years of age. The clinical trial data demonstrate that the safety of intravenous moxifloxacin hydrochloride in patients aged 65 or older was similar to that of comparator-treated patients. In general, elderly patients may be more susceptible to drug-associated effects of the QT interval. Therefore, moxifloxacin hydrochloride should be avoided in patients taking drugs that can result in prolongation of the QT interval (for example, class IA or class III antiarrhythmics) or in patients with risk factors for torsade de pointes (for example, known QT prolongation, uncorrected hypokalemia) [see WARNINGS AND PRECAUTIONS (5.6), DRUG INTERACTIONS (7.5), and CLINICAL PHARMACOLOGY (12.3)].
- NONCLINICAL TOXICOLOGY
- Animal Toxicology and/or Pharmacology
- A QT-prolonging effect of moxifloxacin was found in dog studies, at plasma concentrations about five times the human therapeutic level. The combined infusion of sotalol, a Class III antiarrhythmic agent, with moxifloxacin induced a higher degree of QTc prolongation in dogs than that induced by the same dose (30 mg/kg) of moxifloxacin alone. Electrophysiological in vitro studies suggested an inhibition of the rapid activating component of the delayed rectifier potassium current (IKr) as an underlying mechanism.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
251
23841
Other ADRs
56248
38325339
Odds Ratio = 7.174
Drug Property Information
ATC Code(s):
- J01MA14 - moxifloxacin hydrochloride
- J01MA1 -
- J01MA - Fluoroquinolones
- J01M - QUINOLONE ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- S01AE07 - moxifloxacin hydrochloride
- S01AE -
- S01A - ANTIINFECTIVES
- S01 - OPHTHALMOLOGICALS
- S - SENSORY ORGANS
Active Ingredient:MOXIFLOXACIN HYDROCHLORIDE
Active Ingredient UNII:C53598599T
Drugbank ID:DB00218
PubChem Compound:152946
CTD ID: D000077266
PharmGKB:PA450555
CAS Number:151096-09-2
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 400.0 mg/day J01MA14
Chemical Structure: 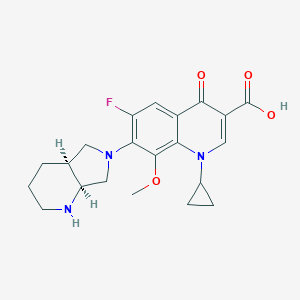

SMILE Code:
[H][C@]12CN(C[C@@]1([H])NCCC2)C1=C(F)C=C2C(=O)C(=CN(C3CC3)C2=C1OC)C(O)=O
[H][C@]12CN(C[C@@]1([H])NCCC2)C1=C(F)C=C2C(=O)C(=CN(C3CC3)C2=C1OC)C(O)=O
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.