Search for drugs:
Typing the drug name to query
RUXOLITINIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effects on QT Interval
- At a concentration 23 times the Cmax of the maximum recommended dose, lomitapide does not prolong QTc to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
9
24083
Other ADRs
124959
38256628
Odds Ratio = 0.115
Drug Property Information
ATC Code(s):
- L01EJ01 - ruxolitinib
- L01EJ -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:RUXOLITINIB
Active Ingredient UNII:82S8X8XX8H
Drugbank ID:DB08877
PubChem Compound:25126798
CTD ID: C540383
PharmGKB:PA166123386
CAS Number:941678-49-5
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 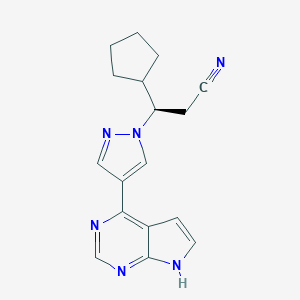

SMILE Code:
N#CC[C@H](C1CCCC1)N1C=C(C=N1)C1=C2C=CNC2=NC=N1
N#CC[C@H](C1CCCC1)N1C=C(C=N1)C1=C2C=CNC2=NC=N1
Reference
1: Pharmacogenomics of COVID-19 therapies.
[Takahashi Takuto,Luzum Jasmine A,Nicol Melanie R,Jacobson Pamala A]NPJ Genom Med,2020 Aug 18;5:35. PMID: 32864162
2: Outcomes of patients with myelofibrosis treated with compassionate use pacritinib: a sponsor-independent international study.
[Mascarenhas J,Virtgaym E,Stal M,Blacklock H,Gerds A T,Mesa R,Ganly P,Snyder D,Tabbara I,Tremblay D,Moshier E]Ann Hematol,2018 Aug;97(8):1369-1374. PMID: 29616317
3: Evaluation of the effect of ruxolitinib on cardiac repolarization: A thorough QT study.
[Punwani Naresh,Yeleswaram Swamy,Chen Xuejun,Bowman Jill,Soloviev Maxim,Williams William]Clin Pharmacol Drug Dev,2014 May;3(3):207-14. PMID: 27128611
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.