Search for drugs:
Typing the drug name to query
PRETOMANID
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- QT prolongation was reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see WARNINGS AND PRECAUTIONS (5.1), ADVERSE REACTIONS (6.1), and CLINICAL PHARMACOLOGY (12.2)]. QT prolongation is a known adverse reaction of bedaquiline. Obtain an ECG before initiation of treatment, and at least 2, 12, and 24 weeks after starting treatment with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Obtain serum potassium, calcium, and magnesium at baseline and correct if abnormal. Monitor these electrolytes if QT prolongation is detected [see ADVERSE REACTIONS (6.1)].
- The following may increase the risk for QT prolongation when patients are receiving bedaquiline as part of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid: a history of Torsade de Pointes, congenital long QT syndrome, ongoing hypothyroidism, ongoing bradyarrhythmia, uncompensated heart failure, or serum calcium, magnesium, or potassium levels below the lower limits of normal. If necessary, bedaquiline treatment initiation could be considered in these patients after a favorable benefit-risk assessment and with frequent ECG monitoring.
- Discontinue the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid if the patient develops clinically significant ventricular arrhythmia or a QTcF interval of greater than 500 ms (confirmed by repeat ECG). If syncope occurs, obtain an ECG to detect QT prolongation.
- OVERDOSAGE
- There is no experience with the treatment of acute overdose with pretomanid. Take general measures to support basic vital functions including monitoring of vital signs and ECG (QT interval) in case of deliberate or accidental overdose.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- A randomized, double-blind, placebo- and positive-controlled (moxifloxacin 400 mg), crossover, thorough QT study of pretomanid was performed in 74 healthy adult subjects. At 400 mg (2 times the approved recommended dosage) and 1,000 mg (5 times the approved recommended dosage) single doses of pretomanid, no significant QT prolongation effect was detected.
- In Study 1, patients received the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for 6 months. No patient had QTcF intervals greater than 480 msec, and 1 subject had a post-baseline increase of QTcF of greater than 60 msec.
- MEDICATION GUIDE
- Heart rhythm problem called QT prolongation. The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can cause a heart rhythm problem. Heart rhythm problem is a side effect of bedaquiline. Your healthcare provider should do a test called an electrocardiogram (ECG) to check your heart before you start taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid and at least 2, 12, and 24 weeks after starting treatment.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- J04AK08 - pretomanid
- J04AK - Other drugs for treatment of tuberculosis
- J04A - DRUGS FOR TREATMENT OF TUBERCULOSIS
- J04 - ANTIMYCOBACTERIALS
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:Pretomanid
Active Ingredient UNII:2XOI31YC4N
Drugbank ID:DB05154
PubChem Compound:456199
CTD ID: C410767
CAS Number:187235-37-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 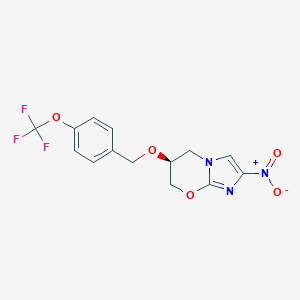

SMILE Code:
[O-][N+](=O)C1=CN2C[C@@H](COC2=N1)OCC1=CC=C(OC(F)(F)F)C=C1
[O-][N+](=O)C1=CN2C[C@@H](COC2=N1)OCC1=CC=C(OC(F)(F)F)C=C1
Reference
1: {'i': 'Rv0678', '#text': 'Comparative Efficacy of the Novel Diarylquinoline TBAJ-587 and Bedaquiline against a Resistant Mutant in a Mouse Model of Tuberculosis.'}
[Xu Jian,Converse Paul J,Upton Anna M,Mdluli Khisimuzi,Fotouhi Nader,Nuermberger Eric L]Antimicrob Agents Chemother,2021 Mar 18;65(4):e02418-20. PMID: 33526488
2: Long-Term Effects on QT Prolongation of Pretomanid Alone and in Combinations in Patients with Tuberculosis.
[Li Hanbin,Salinger David H,Everitt Daniel,Li Mengchun,Del Parigi Angelo,Mendel Carl,Nedelman Jerry R]Antimicrob Agents Chemother,2019 Sep 23;63(10):e00445-19. PMID: 31358590
3: Clinical Implications of New Drugs and Regimens for the Treatment of Drug-resistant Tuberculosis.
[Kwon Yong-Soo]Chonnam Med J,2017 May;53(2):103-109. PMID: 28584788
4: Synthetic investigational new drugs for the treatment of tuberculosis.
[Kwon Yong-Soo,Koh Won-Jung]Expert Opin Investig Drugs,2016;25(2):183-93. PMID: 26576631
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.