Search for drugs:
Typing the drug name to query
LAPATINIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- A concentration-dependent QT prolongation has been associated with TYKERB [see Clinical Pharmacology (12.2)]. Monitor patients who have or may develop prolongation of QTc during treatment with TYKERB. These conditions include patients with hypokalemia or hypomagnesemia, with congenital long QT syndrome, patients taking antiarrhythmic medicines or other medicinal products with known risk for QT prolongation/ Torsades de Pointes (TdP), and cumulative high-dose anthracycline therapy. Correct hypokalemia or hypomagnesemia prior to TYKERB administration.
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of TYKERB. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiac Disorders: Ventricular arrhythmias/Torsades de Pointes (TdP). Electrocardiogram (ECG) QT prolongation.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of lapatinib on the QT-interval was evaluated in a single-blind, placebo-controlled, single sequence (placebo and active treatment) crossover study in patients with advanced solid tumors (N = 58). During the 4-day treatment period, three doses of matching placebo were administered 12 hours apart in the morning and evening on Day 1 and in the morning on Day 2. This was followed by three doses of lapatinib 2000 mg (1.3 – 1.6 times the recommended dosage) administered in the same way. Measurements, including ECGs and pharmacokinetic samples were done at baseline and at the same time points on Day 2 and Day 4. In the evaluable population of subjects who had complete dosing and ECG assessments (N = 37), the maximum mean ΔΔQTcF (90% CI) of 8.75 ms (4.08, 13.42) was observed 10 hours after ingestion of the third dose of lapatinib 2000 mg. The ΔΔQTcF exceeded the 5 ms threshold and the upper bound 90% CIs exceeded the 10 ms threshold at multiple time points.
- There was a concentration-dependent increase in QTcF effects [see Warnings and Precautions (5.6)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
19
24073
Other ADRs
34006
38347581
Odds Ratio = 0.891
Drug Property Information
ATC Code(s):
- L01EH01 - lapatinib
- L01EH -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:LAPATINIB DITOSYLATE
Active Ingredient UNII:G873GX646R
Drugbank ID:DB01259
PubChem Compound:208908
CTD ID:D000077341
PharmGKB:PA152241907
CAS Number:231277-92-2
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 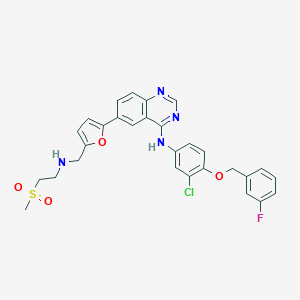

SMILE Code:
CS(=O)(=O)CCNCC1=CC=C(O1)C1=CC2=C(C=C1)N=CN=C2NC1=CC(Cl)=C(OCC2=CC(F)=CC=C2)C=C1
CS(=O)(=O)CCNCC1=CC=C(O1)C1=CC2=C(C=C1)N=CN=C2NC1=CC(Cl)=C(OCC2=CC(F)=CC=C2)C=C1
Reference
1: Differential Inhibitory Actions of Multitargeted Tyrosine Kinase Inhibitors on Different Ionic Current Types in Cardiomyocytes.
[Chang Wei-Ting,Liu Ping-Yen,Lee Kaisen,Feng Yin-Hsun,Wu Sheng-Nan]Int J Mol Sci,2020 Feb 29;21(5):1672. PMID: 32121388
2: Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities.
[Chaar Maher,Kamta Jeff,Ait-Oudhia Sihem]Onco Targets Ther,2018 Sep 25;11:6227-6237. PMID: 30288058
3: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
4: Evaluation of cardiac safety of lapatinib therapy for ErbB2-positive metastatic breast cancer: a single center experience.
[Dogan Erkan,Yorgun Hikmet,Petekkaya Ibrahim,Ozer Necla,Altundag Kadri,Ozisik Yavuz]Med Oncol,2012 Dec;29(5):3232-9. PMID: 22729366
5: Electrophysiological effects of the anti-cancer drug lapatinib on cardiac repolarization.
[Lee Hyang-Ae,Kim Eun-Joo,Hyun Sung-Ae,Park Sung-Gurl,Kim Ki-Suk]Basic Clin Pharmacol Toxicol,2010 Jul;107(1):614-8. PMID: 20406211
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.