Search for drugs:
Typing the drug name to query
VENLAFAXINE HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Use in Patients With Heart Disease
- Premarketing experience with venlafaxine in patients with concomitant systemic illness is limited.
- Caution is advised in administering venlafaxine hydrochloride extended-release tablets to patients with diseases or conditions that could affect hemodynamic responses.
- Venlafaxine has not been evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were systematically excluded from many clinical studies during venlafaxine's premarketing testing. The electrocardiograms were analyzed for 275 patients who received venlafaxine hydrochloride extended-release capsules and 220 patients who received placebo in 8- to 12-week double-blind, placebo-controlled trials in major depressive disorder as well as for 195 patients who received venlafaxine hydrochloride extended-release capsules and 228 patients who received placebo in 12-week double-blind, placebo-controlled trials in Social Anxiety Disorder. The mean change from baseline in corrected QT interval (QTc) for patients treated with venlafaxine hydrochloride extended-release capsules in major depressive disorder studies was increased relative to that for placebo-treated patients (increase of 4.7 msec for venlafaxine hydrochloride extended-release capsules and decrease of 1.9 msec for placebo). The mean change from baseline in QTc for patients treated with venlafaxine hydrochloride extended-release capsules in the Social Anxiety Disorder studies was increased relative to that for placebo-treated patients (increase of 2.8 msec for venlafaxine hydrochloride extended-release capsules and decrease of 2.0 msec for placebo).
- OVERDOSAGE
- Human Experience
- Among the patients included in the premarketing evaluation with venlafaxine hydrochloride immediate-release tablets, there were 14 reports of acute overdose with venlafaxine, either alone or in combination with other drugs and/or alcohol. The majority of the reports involved ingestion in which the total dose of venlafaxine taken was estimated to be no more than several-fold higher than the usual therapeutic dose. The 3 patients who took the highest doses were estimated to have ingested approximately 6.75 g, 2.75 g, and 2.5 g. The resultant peak plasma levels of venlafaxine for the latter 2 patients were 6.24 and 2.35 μg/mL, respectively, and the peak plasma levels of O‑desmethylvenlafaxine were 3.37 and 1.30 μg/mL, respectively. Plasma venlafaxine levels were not obtained for the patient who ingested 6.75 g of venlafaxine. All 14 patients recovered without sequelae. Most patients reported no symptoms. Among the remaining patients, somnolence was the most commonly reported symptom. The patient who ingested 2.75 g of venlafaxine was observed to have 2 generalized convulsions and a prolongation of QTc to 500 msec, compared with 405 msec at baseline. Mild sinus tachycardia was reported in 2 of the other patients.
- In postmarketing experience, overdose with venlafaxine has occurred predominantly in combination with alcohol and/or other drugs. The most commonly reported reactions in overdosage include tachycardia, changes in level of consciousness (ranging from somnolence to coma), mydriasis, seizures, and vomiting. Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), ventricular tachycardia, bradycardia, hypotension, rhabdomyolysis, vertigo, liver necrosis, serotonin syndrome, and death have been reported.
- ADVERSE REACTIONS
- Post-Marketing Experience
- Voluntary reports of other adverse reactions temporally associated with the use of venlafaxine have been received since market introduction. Because these reactions are reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports include the following reactions: agranulocytosis, anaphylaxis, aplastic anemia, catatonia, congenital anomalies, impaired coordination and balance, CPK increased, deep vein thrombophlebitis, delirium, Takotsubo cardiomyopathy, EKG abnormalities such as QT prolongation; cardiac arrhythmias including atrial fibrillation, supraventricular tachycardia, ventricular extrasystoles, and rare reports of ventricular fibrillation and ventricular tachycardia, including torsade de pointes; toxic epidermal necrolysis/Stevens-Johnson Syndrome, erythema multiforme, extrapyramidal symptoms (including dyskinesia and tardive dyskinesia), angle-closure glaucoma, hemorrhage (including eye and gastrointestinal bleeding), hepatic reactions (including GGT elevation; abnormalities of unspecified liver function tests; liver damage, necrosis, or failure; and fatty liver), interstitial lung disease, involuntary movements, LDH increased, neuroleptic malignant syndrome-like reactions (including a case of a 10-year-old who may have been taking methylphenidate, was treated and recovered), neutropenia, night sweats, pancreatitis, pancytopenia, panic, prolactin increased, renal failure, rhabdomyolysis, serotonin syndrome, shock-like electrical sensations or tinnitus (in some cases, subsequent to the discontinuation of venlafaxine or tapering of dose), and syndrome of inappropriate antidiuretic hormone secretion (usually in the elderly).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
284
23808
Other ADRs
88621
38292966
Odds Ratio = 5.155
Drug Property Information
ATC Code(s):
- N06AX16 - venlafaxine hydrochloride
- N06AX - Other antidepressants
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:VENLAFAXINE
Active Ingredient UNII:GRZ5RCB1QG
Drugbank ID:DB00285
PubChem Compound:5656
CTD ID:D000069470
PharmGKB:PA451866
CAS Number:93413-69-5
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 100.0 mg/day N06AX16
Chemical Structure: 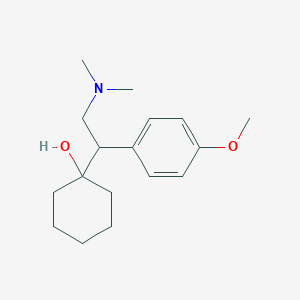

SMILE Code:
COC1=CC=C(C=C1)C(CN(C)C)C1(O)CCCCC1
COC1=CC=C(C=C1)C(CN(C)C)C1(O)CCCCC1
Reference
1: QTc Time Correlates with Amitriptyline and Venlafaxine Serum Levels in Elderly Psychiatric Inpatients.
[Hefner Gudrun,Hahn Martina,Hohner Matthias,Roll Sybille C,Klimke Ansgar,Hiemke Christoph]Pharmacopsychiatry,2019 Jan;52(1):38-43. PMID: 29466824
2: Association of QT-Prolonging Medication Use in CKD with Electrocardiographic Manifestations.
[Snitker Soren,Doerfler Rebecca M,Soliman Elsayed Z,Deo Rajat,St Peter Wendy L,Kramlik Susan,Fischer Michael J,Navaneethan Sankar,Delafontaine Patrice,Jaar Bernard G,Ojo Akinlolu,Makos Gail K,Slaven Anne,Weir Matthew R,Zhan Min,Fink Jeffrey C,for CRIC Study Investigators]Clin J Am Soc Nephrol,2017 Sep 7;12(9):1409-1417. PMID: 28793999
3: Venlafaxine and takotsubo syndrome: Can we learn more from published patient cases?
[Madias John E]Int J Cardiol,2016 Dec 15;225:73-74. PMID: 27716552
4: Venlafaxine induced QTc interval prolongation in a therapeutic dose.
[Bavle Amar]Asian J Psychiatr,2015 Aug;16:63-4. PMID: 26187237
5: QT interval prolongation associated with venlafaxine administration.
[Letsas Konstantinos,Korantzopoulos Panagiotis,Pappas Loucas,Evangelou Dimitrios,Efremidis Michalis,Kardaras Fotis]Int J Cardiol,2006 Apr 28;109(1):116-7. PMID: 16574528
6: Comparative toxicity of citalopram and the newer antidepressants after overdose.
[Kelly C A,Dhaun N,Laing W J,Strachan F E,Good A M,Bateman D N]J Toxicol Clin Toxicol,2004;42(1):67-71. PMID: 15083939
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.