Search for drugs:
Typing the drug name to query
TACROLIMUS
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- Tacrolimus may prolong the QT/QTc interval and may cause Torsade de Pointes. Avoid tacrolimus in patients with congenital long QT syndrome. In patients with congestive heart failure, bradyarrhythmias, those taking certain antiarrhythmic medications or other medicinal products that lead to QT prolongation, and those with electrolyte disturbances such as hypokalemia, hypocalcemia, or hypomagnesemia, consider obtaining electrocardiograms and monitoring electrolytes (magnesium, potassium, calcium) periodically during treatment.
- When co-administering tacrolimus with other substrates and/or inhibitors of CYP3A4 that also have the potential to prolong the QT interval, a reduction in tacrolimus dose, frequent monitoring of tacrolimus whole blood concentrations, and monitoring for QT prolongation is recommended. Use of tacrolimus with amiodarone has been reported to result in increased tacrolimus whole blood concentrations with or without concurrent QT prolongation [see Drug Interactions (7)].
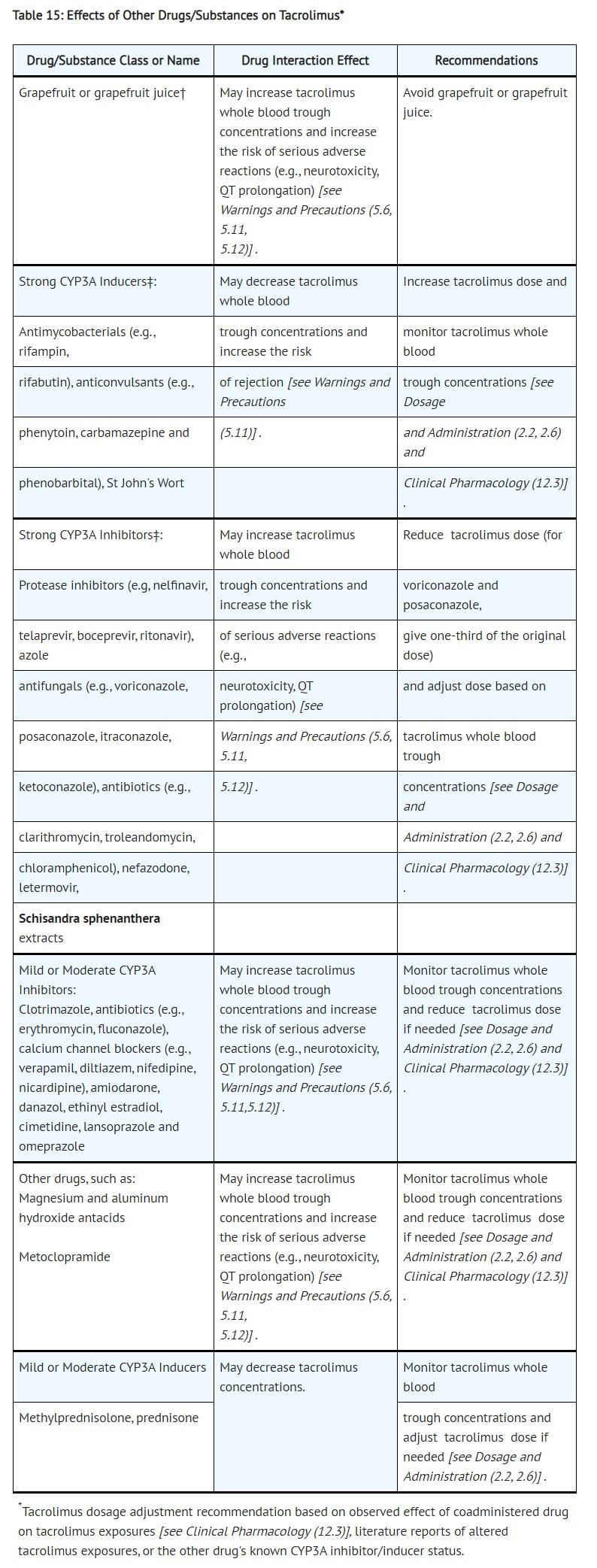
- DRUG INTERACTIONS
- Effects of Other Drugs on Tacrolimus
- ADVERSE REACTIONS
- Postmarketing Adverse Reactions
- The following adverse reactions have been reported from worldwide marketing experience with tacrolimus. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of the reporting, or (3) strength of causal connection to the drug.
- Cardiovascular: Atrial fibrillation, atrial flutter, cardiac arrhythmia, cardiac arrest, electrocardiogram T wave abnormal, flushing, myocardial infarction, myocardial ischemia, pericardial effusion, QT prolongation, Torsade de Pointes, venous thrombosis deep limb, ventricular extrasystoles, ventricular fibrillation, myocardial hypertrophy [see Warnings and Precautions (5.13)].
- PATIENT PACKAGE INSERT
- Tacrolimus capsules may cause serious side effects, including:
- changes in the electrical activity of your heart (QT prolongation).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
50
24042
Other ADRs
135842
38245745
Odds Ratio = 0.586
Drug Property Information
ATC Code(s):
- L04AD02 - tacrolimus
- L04AD - Calcineurin inhibitors
- L04A - IMMUNOSUPPRESSANTS
- L04 - IMMUNOSUPPRESSANTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
- D11AH01 - tacrolimus
- D11AH -
- D11A - OTHER DERMATOLOGICAL PREPARATIONS
- D11 - OTHER DERMATOLOGICAL PREPARATIONS
- D - DERMATOLOGICALS
Active Ingredient:TACROLIMUS
Active Ingredient UNII:WM0HAQ4WNM
Drugbank ID:DB00864
PubChem Compound:445643
CTD ID:D016559
PharmGKB:PA451578
CAS Number:104987-11-3
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 5.0 mg/day L04AD02
Chemical Structure: 

SMILE Code:
CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC
CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC
Reference
1: Risk of QT prolongation through drug interactions between hydroxychloroquine and concomitant drugs prescribed in real world practice.
[Choi Byung Jin,Koo Yeryung,Kim Tae Young,Chung Wou Young,Jung Yun Jung,Park Ji Eun,Lim Hong-Seok,Park Bumhee,Yoon Dukyong]Sci Rep,2021 Mar 25;11(1):6918. PMID: 33767276
2: Hydroxychloroquine and maintenance immunosuppression use in kidney transplant recipients: Analysis of linked US registry and claims data.
[Lentine Krista L,Lam Ngan N,Caliskan Yasar,Alhamad Tarek,Xiao Huiling,Schnitzler Mark A,Chang Su-Hsin,Axelrod David,Segev Dorry L,McAdams-DeMarco Mara,Kasiske Bertram L,Hess Gregory P,Brennan Daniel C]Clin Transplant,2020 Dec;34(12):e14118. PMID: 33048372
3: Posaconazole liquid vs tablet formulation in lung transplant recipients.
[Stelzer D,Weber A,Ihle F,Matthes S,Ceelen F,Zimmermann G,Kneidinger N,Schramm R,Winter H,Zoller M,Vogeser M,Behr J,Neurohr C]Mycoses,2018 Mar;61(3):186-194. PMID: 29110351
4: Sublingual administration improves systemic exposure of tacrolimus in kidney transplant recipients: comparison with oral administration.
[Federico Stefano,Carrano Rosa,Sabbatini Massimo,Nappi Riccardo,Russo Luigi,Apicella Luca,Balletta Mario Maria,Santangelo Michele,Mosca Teresa,Tarantino Giovanni,Capone Domenico]Eur J Clin Invest,2016 Jul;46(7):651-7. PMID: 27240092
5: Impact of pre-implant amiodarone exposure on outcomes in cardiac transplant recipients.
[Jennings Douglas L,Martinez Brandon,Montalvo Sheila,Lanfear David E]Heart Fail Rev,2015 Sep;20(5):573-8. PMID: 25925244
6: Case report: drug interaction between tacrolimus and amiodarone with QT prolongation.
[Burger Cathy I,Clase Catherine M,Gangji Azim S]Transplantation,2010 May 15;89(9):1166-7. PMID: 20440199
7: Clinically relevant QTc prolongation due to overridden drug-drug interaction alerts: a retrospective cohort study.
[van der Sijs Heleen,Kowlesar Ravi,Klootwijk A Peter J,Nelwan Stefan P,Vulto Arnold G,van Gelder Teun]Br J Clin Pharmacol,2009 Mar;67(3):347-54. PMID: 19523015
8: Unintended consequences of reducing QT-alert overload in a computerized physician order entry system.
[van der Sijs Heleen,Kowlesar Ravi,Aarts Jos,Berg Marc,Vulto Arnold,van Gelder Teun]Eur J Clin Pharmacol,2009 Sep;65(9):919-25. PMID: 19415251
9: Frequency-dependent and proarrhythmogenic effects of FK-506 in rat ventricular cells.
[Fauconnier Jérémy,Lacampagne Alain,Rauzier Jean-Michel,Fontanaud Pierre,Frapier Jean-Marc,Sejersted Ole M,Vassort Guy,Richard Sylvain]Am J Physiol Heart Circ Physiol,2005 Feb;288(2):H778-86. PMID: 15471978
10: Prolonged cardiac repolarization after tacrolimus and haloperidol administration in the critically ill patient.
[Akers Wendell S,Flynn Jeremy D,Davis George A,Green Amy E,Winstead P Shane,Strobel Gunnar]Pharmacotherapy,2004 Mar;24(3):404-8. PMID: 15040655
11: Quantitative relationship between myocardial concentration of tacrolimus and QT prolongation in guinea pigs: pharmacokinetic/pharmacodynamic model incorporating a site of adverse effect.
[Minematsu T,Ohtani H,Yamada Y,Sawada Y,Sato H,Iga T]J Pharmacokinet Pharmacodyn,2001 Dec;28(6):533-54. PMID: 11999291
12: Pharmacokinetic/pharmacodynamic analysis of tacrolimus-induced QT prolongation in guinea pigs.
[Minematsu T,Ohtani H,Sato H,Iga T]Biol Pharm Bull,1999 Dec;22(12):1341-6. PMID: 10746167
13: Sustained QT prolongation induced by tacrolimus in guinea pigs.
[Minematsu T,Ohtani H,Sato H,Iga T]Life Sci,1999;65(14):PL197-202. PMID: 10530807
14: QT prolongation and near fatal cardiac arrhythmia after intravenous tacrolimus administration: a case report.
[Hodak S P,Moubarak J B,Rodriguez I,Gelfand M C,Alijani M R,Tracy C M]Transplantation,1998 Aug 27;66(4):535-7. PMID: 9734501
15: Systemic antifungal agents. Drug interactions of clinical significance.
[Albengres E,Le Louët H,Tillement J P]Drug Saf,1998 Feb;18(2):83-97. PMID: 9512916
16: QT prolongation and Torsades de Pointes after administration of FK506.
[Johnson M C,So S,Marsh J W,Murphy A M]Transplantation,1992 Apr;53(4):929-30. PMID: 1373538
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.